To Issue 142
Citation: McGowan M, “Enabling Large-Volume, High-Dose Subcutaneous Autoinjections with Cartridge-Based Technologies”. ONdrugDelivery, Issue 142 (Feb 2023), pp 96–99.
Michael McGowan, examines the emerging interest in autoinjectors that are capable of delivering higher injection volumes into the subcutaneous space and considers the potential benefits of SHL Medical’s cartridge-based 3 and 5 mL Maggie® autoinjectors.
An examination of the clinical pipeline reveals the extent to which oncology, in particular immuno-oncology, features heavily in the biotech industry’s research and development focus. With groundbreaking advances in the understanding of cancer, new therapies and molecules currently account for around 39% of the industry’s development pipeline.1 Traditionally delivered via intravenous infusion within a hospital setting, molecules used for oncology indications have historically been well outside the scope for autoinjectors or other subcutaneous drug delivery devices. However, with the advent of injection site absorption enhancers and other formulation innovations, the pharmaceutical industry’s interest in more practical and patient-centric administration for oncology drugs has grown.
“Recent years have seen the launch of a number of oncology treatments formulated for subcutaneous administration in a variety of injection volumes, ranging from 5 mL to around 20 mL.”
Recent years have seen the launch of a number of oncology treatments formulated for subcutaneous administration in a variety of injection volumes, ranging from 5 mL to around 20 mL. Within the drug delivery field, on-body devices for higher-volume subcutaneous drugs have generated a great deal of interest in recent years and a number of different models and concepts have been well presented and promoted by device companies. However, to date, the number of commercially available drugs in such devices remains low, suggesting that pharmaceutical companies have still not fully embraced the concept of on-body devices for higher-volume injections.
THE RISE OF THE AUTOINJECTOR AND THE TREND TOWARDS HIGHER VOLUMES
Since the mid-2000s, the number of drugs commercially available in single-use autoinjectors has risen considerably. While it is difficult to be exact on the number of marketed drugs in autoinjectors, it is now thought to be well in excess of 90 brands, with global sales close to 300 million units.2 This growth has occurred mainly due to the rise of chronic conditions associated with changing demographics and the advent of biologics to treat conditions such as Type 2 diabetes, rheumatoid arthritis, psoriasis, atopic dermatitis and, more recently, obesity. The development of these highly effective biologics in conjunction with autoinjectors has seen long-term treatment shift from a clinical setting into patients’ homes.
To date, the overwhelming majority of the drugs commercialised in autoinjectors have injection volumes of 1 mL or less and use a prefilled syringe (PFS) with stakedneedle as the primary container for the drug. Over the past few years, a number of drugs with higher injection volumes have reached the market in autoinjectors that are designed around 2.25 mL PFSs. Treating atopic dermatitis, high cholesterol and other chronic medical conditions, these drugs and devices are showing that higher-volume subcutaneous injections with autoinjectors are possible in a home setting. As handheld single-use autoinjectors continue to gain traction in new therapy areas, the perception of the higher volume potential of the subcutaneous space also continues to evolve.
DEVELOPING CARTRIDGE-BASED SOLUTIONS IN A PREDOMINANTLY PFS-BASED AUTOINJECTOR MARKET
Since the company’s creation more than 30 years ago, SHL Medical has been at the forefront of autoinjector innovation and industrialisation on a large scale. Based on experience in developing and commercialising projects, SHL Medical understands that each device project is unique and requires a tight collaboration between the device supplier and the drug company – whether the project is based on a platform technology, such as SHL Medical’s Molly®, or on highly customised and bespoke designs.
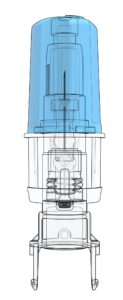
Figure 1: A close-up image of SHL Medical’s proprietary NIT technology. The NIT subassembly is a sterile unit that houses the needle within the cartridge-based Maggie autoinjector.
Responding to specific customer needs has led SHL Medical to develop autoinjector designs that not only accommodate PFSs with staked needles but also cartridges. Well-known and trusted as a primary container within the pharmaceutical industry, cartridges have a number of properties that are well adapted to the needs of sensitive biologic molecules, such as baked silicone, absence of tungsten and limited contact materials.4–8 As the drug/primary container contact area increases with higher volumes, these properties may become more significant. Through innovative device technology working in tandem with primary container system design, SHL Medical is looking to drive forward the evolution of the large-volume, high-dose autoinjector market segment.
SHL MEDICAL’S MAGGIE® – A TWO-STEP CARTRIDGE-BASED AUTOINJECTOR FOR LARGER VOLUMES
The use of cartridges as the primary drug container requires a highly innovative solution to facilitate an end-user experience that is equivalent to that of a two-step autoinjector built around a PFS with staked needle. The result is SHL Medical’s Maggie® autoinjector, which is designed to accommodate a standard 3 mL cartridge as the drug primary container. The Maggie device incorporates a cannula unit (Figure 1) featuring SHL Medical’s Needle Isolation Technology (NIT®). NIT offers the added flexibility of a wide range of needle lengths and gauges not readily available in PFSs with staked needles.
SHL MEDICAL’S NIT® TECHNOLOGY
SHL Medical’s NIT enables cartridges to be used as the primary container in a simple two-step autoinjector. The NIT unit consists of a sterile self-contained cannula with patient and non-patient ends. As the needle cap is removed, the non-patient end moves backwards to pierce the cartridge septum and open the drug fluid path (Figure 2). The patient end of the needle remains hidden at all times by a sliding needle cover, which also serves to activate the autoinjector when pushed against the injection site.
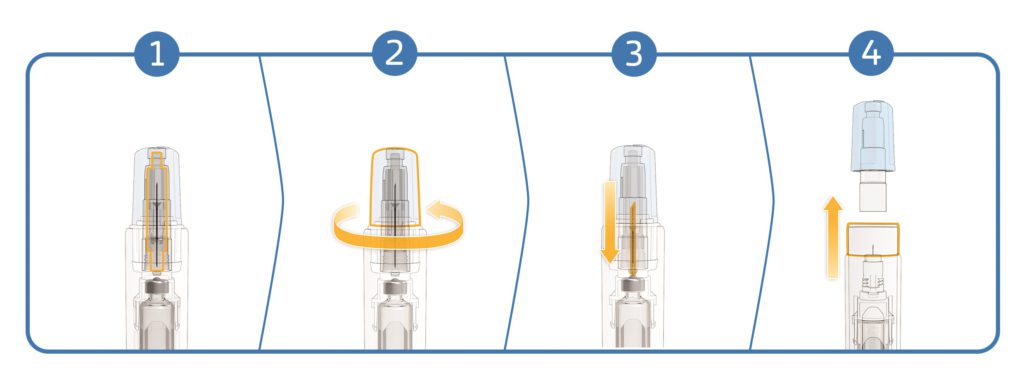
Figure 2: An overview of the NIT mechanism: 1. The closed system. 2. Twist off the needle cap. 3. The non-patient end of the cannula moves backwards to pierce the cartridge septum and open the fluid path. 4. Remove the needle cap to ready the device for injection by pushing against the skin.
“The NIT – created with end user safety and convenience considerations in mind – eliminates the need for patients to manually attach the needle to the cartridge prior to injection.”
THE “SPACE BETWEEN” – CARTRIDGE BIOBURDEN VERIFICATION
The NIT – created with end user safety and convenience considerations in mind – eliminates the need for patients to manually attach the needle to the cartridge prior to injection. This simplifies the whole injection process while underscoring the interconnected importance of patient safety and convenience. Traditionally, some cartridge-based injection systems recommend swabbing the cartridge septum as a precaution, with the aim of reducing the surface bioburden prior to injection. In the case of NIT, its end-user-friendly architecture results in a septum that is neither visible nor accessible prior to the use of the Maggie autoinjector.
Nevertheless, as any drug product may be susceptible to bacterial contamination when the septum is pierced, SHL Medical sought to understand this question fully by conducting a multi-phase bioburden study using NIT. For the device-specific verification phase of the study, autoinjector devices assembled at SHL’s facilities in Deerfield Beach (FL, US) for performance qualification (PQ) and process validation (PV) activities were subjected to bioburden testing. None of the PQ or PV samples showed any evidence of microbial growth.
These results provide industry evidence of contaminant-free dose delivery using the market-proven, cartridge-based NIT.3 Devices integrating the NIT cannula system have already enjoyed considerable commercial success on the market, recording end-user sales of several tens of millions since launch in 2017.1 Hence, bioburden and the “space between” should not be a concern in the manufacturing of cartridge-based combination products in Maggie devices.
PRIMARY CONTAINER OPTIONS – PFS OR CARTRIDGE
Originally developed decades ago for heparins and vaccines, the PFS has demonstrated itself to be a very successful and highly versatile primary container for the storage and the injection of complex biologic medicines. The integration of PFSs with staked needles into autoinjectors has enabled pharmaceutical companies to develop drug brands that are both clinically and practically efficacious and easy for patients to use.
However, despite the success of PFSs as a primary container for biologics, the stability of protein-based molecules or complex monoclonal antibodies in PFSs with staked needles can still present challenges. It has been widely documented that the presence of silicone oil for lubricating the glass barrel and the presence of tungsten residues from the manufacturing process can potentially pose stability issues for PFS-based combination products.4–6 Other contact materials, such as the adhesive used to bond the staked needle to the barrel and the needle itself, also need to be taken into consideration. In general, when considering biologics, cartridges may present some stability advantages as a primary container overall, as they have fewer materials in direct contact with the drug.
The siliconisation of the primary container is particularly important, as silicone oil-water and air-water interfaces can give rise to protein aggregation and particle formation in the drug product.4 Baking the silicone using high temperatures has been shown to reduce silicone migration and protein aggregation efficiently, while maintaining the functionality of the injection system.7 As reported by Funke et al, baking silicone on parenteral containers is a challenging process that may require temperatures in excess of 300°C to obtain uniform silicone layers.8 Considering the need to preserve the bonding force between the staked needle and the glass barrel, it would not be desirable to expose a PFS to such temperatures. However, cartridges, which do not have a staked needle, are well suited to undergo such a process as a way to reduce protein aggregation and particle formation.
MAGGIE 3 AND 5 ML – HIGHER-VOLUME AUTOINJECTORS FOR EMERGING NEEDS
Since the approval and commercial availability of the first drugs with a 2 mL fill volume in SHL Medical’s Molly 2.25 mL autoinjectors, the self-injection of higher volumes has become a reality. As the perception of large-volume subcutaneous injections evolves, the cartridge-based Maggie 3.0 mL has further extended the potential fill volumes for SHL Medical autoinjectors up to 3 mL.
Enabling higher dose delivery and the possibility of less frequent injections, Maggie 3.0 mL provides potential benefits and delivery solutions for both patients and the biotech industry alike. As studies continue to generate more data to suggest that even larger volumes of drug may be administered subcutaneously,9,10 the extension of the Maggie range continues with the introduction of the Maggie 5.0 mL autoinjector.
The compact design and optimised dimensions of the Maggie 5.0 mL (Figure 3) give rise to a device that is not significantly larger than other successful and widely used SHL Medical autoinjectors. With its simple two-step activation, Maggie 5.0 mL integrates SHL Medical’s NIT cannulas and the breadth of possible needle gauge and length options that this technology opens up.
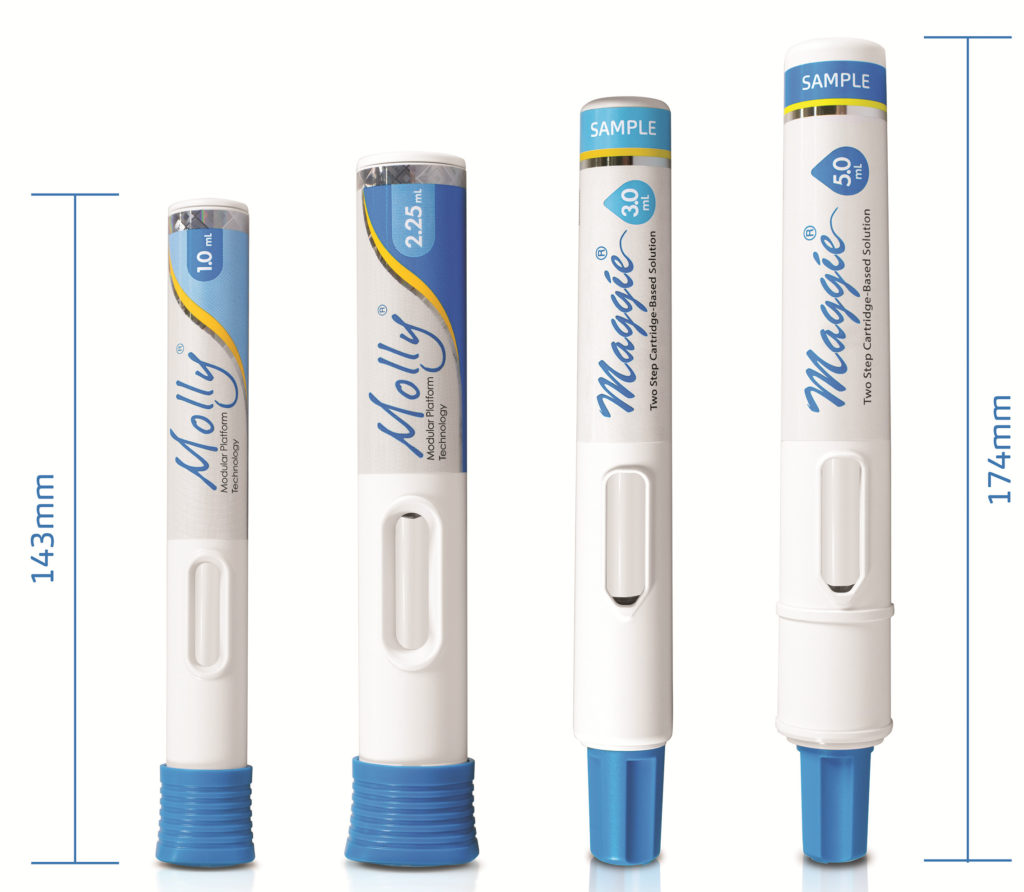
Figure 3: Size comparison between the conventional Molly autoinjectors and the Maggie 3.0 and 5.0 mL devices.
SUPPORTING THE FUTURE OF LARGE-VOLUME, HIGH-DOSE DRUG DELIVERY
With the development of 5 mL cartridge autoinjectors well underway, SHL’s Maggie device aims to support therapy areas that will one day require higher injection volumes, including the emerging trend towards subcutaneous drugs in the oncology market. As the product development process gathers pace, the use of autoinjectors for volumes up to 5 mL, and possibly higher, will require deeper clinical investigation to better understand the influence of injection variables, such as time, volume, viscosity and injection depth. As the conundrums of this new subcutaneous drug delivery area are unravelled, SHL Medical will continue to investigate and explore the intrinsic advantages of cartridge-based technologies while working with its partners around the world to address longstanding questions in large-volume, high-dose drug delivery.
REFERENCES
- Lloyd I, “Pharma R&D Annual Review 2022 – Navigating the Landscape”. Citeline Informa Pharma Intelligence, Mar 2022.
- “MIDAS”. IQVIA dataset, 2022.
- Hartl A, Guimond L, “Addressing Primary Container Challenges in Autoinjector Drug Delivery: Interim Results of a Multiphase Bioburden Study for a Cartridge- Based Autoinjector”. 2022 PDA Universe of Pre-Filled Syringes and Injection Devices Conference presentation, Oct 2022.
- Gerhardt A et al, “Protein aggregation and particle formation in prefilled glass syringes”. J Pharm Sci, 2014, Vol 103(6), pp 1601–1612.
- Bee JS et al, “Precipitation of a monoclonal antibody by soluble tungsten”. J Pharm Sci, 2009, Vol 98(9), pp 3290–3301.
- Jones LS, Kaufmann A, Middaugh CR, “Silicone oil induced aggregation of proteins”. J Pharm Sci, 2005, Vol 94(4), pp 918–927.
- Funke S et al, “Silicone Migration From Baked-on Silicone Layers. Particle Characterization in Placebo and Protein Solutions”. J Pharm Sci, 2016, Vol 105(12), pp 3520–3531.
- Funke S et al, “Optimization of the bake-on siliconization of cartridges. Part II: Investigations into burn-in time and temperature”. Eur J Pharm Biopharm, 2016, Vol 105, pp 209–222.
- Woodley W, “Large Volume Subcutaneous Injection: Feasibility and Acceptability Patterns Across a Sequence of Translational Studies”. 2022 PDA Universe of Pre-Filled Syringes and Injection Devices Conference poster, Oct 2022.
- Badkar AV et al, “Subcutaneous Delivery of High-Dose/Volume Biologics: Current Status and Prospect for Future Advancements”. Drug Des Devel Ther, 2021, Vol 15, pp 159–170.



