Citation: Pizitz T, Mealing D, “130 a Day and Growing: Answering the US Opioid Overdose Crisis”. ONdrugDelivery Magazine, Issue 96 (Apr 2019), pp 46-48.
Todd Pizitz and Donald Mealing discuss the ongoing opioid crisis in the US and the current use of the opioid reversal medication naxalone, and introduce the CounterAct device for naxalone home use.
In the US in 2017, over 47,000 deaths were attributable to opioid-based substances.1 The average number of deaths per day from opioid overdoses, currently around 130, continues to rise. In fact, some researchers project that opioid overdose deaths are estimated to increase annually by 147%, approaching 81,700 by 2025.2 Due to this upward trend in the number of deaths, recent estimates have determined that one is more likely to die from an accidental opioid overdose than from a motor vehicle accident.3
“According to the US Centers for Disease Control and Prevention (CDC), the administration of naloxone from the years 1996 to 2014 assisted in reversing the effects of opioid overdoses in an estimated 26,000 cases.4”
As with any epidemic, researchers, scientists, legislators and interventionists are endeavouring to slow or cure the progression of the life-ending situations and circumstances. Similar efforts occurred in 1981 when the first cases of auto-immune deficiency syndrome (AIDS) began to emerge; the disease was identified and studied, and interventions were created to reduce death rates and treat AIDS and associated syndromes.
The opioid epidemic, too, has been identified and studied, but the interventions or requisite changes have not moved swiftly enough yet to see the death rate drop.
One such intervention aimed at reducing the opioid overdose death rate is increasing the availability of naloxone, which is an opioid reversal medication, approved for public use by the US FDA in 1971. Beginning in 1996, naloxone was more widely marketed, distributed and made available to people who are not medically trained. Naloxone is marketed as a generic and brand names such as Narcan® (ADAPT Pharma, Radnor, PA, US) and Evzio® (kaléo, Richmond, VA, US) have emerged as FDA-approved naloxone products to counter the effects of an opioid overdose.
Evzio, a handheld autoinjector that delivers naloxone IM, was approved in the US in 2014. Narcan, an intranasal naloxone product, was approved in 2015. According to the US Centers for Disease Control and Prevention (CDC), the administration of naloxone from the years 1996 to 2014 assisted in reversing the effects of opioid overdoses in an estimated 26,000 cases.4
Even with these two FDA-approved formulations of naloxone available for public use, the death rate from opioid overdoses has not declined. Recent efforts through legislation, such as the co-prescription bills in states such as California and the US Surgeon General recommending that the public carry naloxone, have not made a significant impact in reducing the deaths associated with opioids.
CounterAct is a small technology startup dedicated to tackling the opioid crisis and reducing the overdose death rate. Its founders have personal experience with the effects of the crisis. The company was founded to realise an idea the founders had in 2017 whilst discussing an FDA innovation challenge on reducing opioid deaths.
“One key way to improve public access to naloxone is to improve its proximity to opioid patients. CounterAct’s device aligns with this priority by placing a unit-dose of life-saving naloxone on top of the container holding an opioid patient’s prescription pills.”
At the core of the idea was how best to improve speed of access to and provide wider availability of life-saving naloxone.
Following its FDA approval, Narcan saw rapid adoption by emergency medical response agencies as a must-have opioid overdose resource. Narcan’s simple nasal spray route of administration, combined with its single unit-dose applicator, provided law enforcement and medical first responders with an extremely compact, efficient and effective intervention tool. Narcan has demonstrated noteworthy success in reversing the effects of an opioid overdose and has commonly been administered by emergency medical responders.
Part of the opioid epidemic is the prescription opioid landscape. Accidental ingestion of opioid medication resulting in an overdose accounts for an estimated 40% of all of the opioid overdose deaths.5 In other words, an estimated 46 people a day have been dying from prescription opioid overdoses. In 2017, the CDC reported that 191,218,272 prescriptions were written for opioid medications in the US. Narcan has, as mentioned previously, been adopted by emergency medical responders, but has not crossed into widespread public use, even with almost 200 million opioid prescriptions written annually.
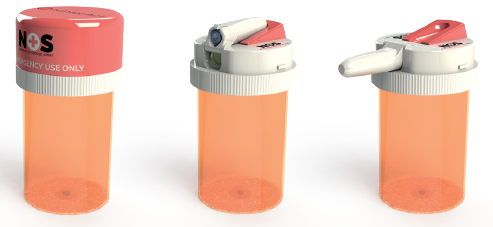
Figure 1: The CounterAct naloxone device fits on top of a standard US prescription pill bottle.
But how then could naloxone’s public availability and time to access be improved over emergency response? CounterAct conceived of an idea to expand upon Narcan’s utility by designing a unique device suited for opioid patient home use. One key way to improve public access to naloxone is to improve its proximity to opioid patients. CounterAct’s device aligns with this priority by placing a unitdose of life-saving naloxone on top of the container holding an opioid patient’s prescription pills (Figure 1). In a suspected overdose emergency, a patient’s family members, friends or associates can instantly administer the naloxone spray after calling the emergency services, thus saving precious time. Additionally, the cautionary effect of having the two medications paired together may prove to increase patient medication compliance to prescriber’s recommendations, offering a reminder that misuse of opioid medication can result in death.
“Having a naloxone nasal spray in the home is akin to having a fire extinguisher in case of fire.”
Specific features the CounterAct device were incorporated for non-emergency, nonmedical trained personnel suited for home use. Having a naloxone nasal spray in the home is akin to having a fire extinguisher in case of fire. In fact, The National Association of Fire Equipment Distributors (NAFED) reported that out of the 13,221 fire incidents reported, fire extinguishers successfully extinguished 12,505 fires, lending strong support for having a fire extinguisher in your residence or place of work.6 Therefore, having immediate access to life-saving medication such as naloxone will likely increase the probability of surviving the effects of an opioid overdose if administered in a timely manner.
From an economic perspective, a recent summary authored by the Council of Economic Advisers revealed the economic burden of the opioid crisis in 2015 was US$504 billion (£384 billion), with the mortality costs consisting of over $428 billion.7 Widespread dissemination of naloxone will reduce the healthcare costs associated with the opioid epidemic.
The CounterAct device provides a practical home-based solution to combat opioid overdose rates. The legislative efforts underway to mandate prescribers to co-prescribe naloxone with opioid medications, such as the aforementioned legislation passed in California in September 2018, will hopefully spur more accessibility of this lifesaving medication, and begin the process of ending the opioid epidemic.
REFERENCES
1. “Overview of the Drug Overdose Epidemic: Behind the Numbers”. US Centers for Disease Control and Prevention, 2018.
2. Chen Q et al, “Prevention of Prescription Opioid Misuse and Projected Overdose Deaths in the United States”. JAMA Netw Open, 2019, Vol 2(2), epub.
3. “For the First Time, We’re More Likely To Die From Accidental Opioid Overdose Than Motor Vehicle Crash”. US National Safety Council, 2019.
4. “The history of naloxone”. Cordant Health Solutions, 2017.
5. Scholl L et al, “Drug and Opioid- Involved Overdose Deaths – United States, 2013–2017”. MMWR Morb Mortal Wkly Rep, 67(5152), pp 1419–1427.
6. “Testimony on Behalf of the Pennsylvania Association of Fire Equipment Distributors”. House & Senate Veterans Affairs & Emergency Preparedness Committees Hearing, 2017.
7. Hagemeier NE, “Introduction to the Opioid Epidemic: The Economic Burden on the Healthcare System and Impact on Quality of Life”. Am J Manag Care, 2018, 24(10 Suppl), S200–S206.

