Citation: Pager A, “8 mm Needle – Improving Subcutaneous Chronic Drug Delivery”. ONdrugDelivery Magazine, Issue 101 (Oct 2019), pp 28-32
Aurelie Pager describes how reducing needle length can improve the risk profile of subcutaneous injections and can make injecting viscous formulations easier and quicker, and highlights BD’s development programme for an innovative shorter needle with ultra-thin walls for prefilled syringes.
CHRONIC DISEASE, UNMET MARKET NEEDS
Chronic diseases are on the rise worldwide1,2 and require regular administration of drug therapies to relieve the patients from their symptoms. Biologics, and more specifically monoclonal antibodies (mAbs), are becoming dominant in the biopharmaceutical pipeline thanks to their success in treating chronic conditions, and biologics are expected to account for ~50% of top-100 drug sales by 2024.3 The parenteral route is commonly used, with >80% of chronic biologics delivered subcutaneously using prefilled syringes.4,5
Great progress and efforts have been made in offering drug delivery systems for chronic diseases over the past decades. However, several market unmet needs persist6 and should be considered when developing new manual drug delivery solutions. They include the need to:
- reduce the injection/needle-related anxiety and pain perception
- reduce the risk of accidental intramuscular (IM) injection
- maintain acceptable injection force and time for high-volume and high-viscosity drugs.
“Needle innovations, including the use of a shorter 8 mm needle, offer solutions to enable the delivery of larger, more viscous drugs, without compromising end-user experience.”
To improve patient quality of life and potentially treatment adherence, reducing injection frequency can be one of the strategies adopted by pharma companies. A way to achieve this could be to increase drug dosage7-9 either by increasing the concentration of the drug and/or by delivering a larger volume. Both options may lead to specific challenges.
Increasing the concentration of a biologic solution consequently increases its viscosity. Viscous and large-volume solutions take more force and/or require more time to perform the injection, which can impact end-user (healthcare providers, caregivers and self-injecting patients) acceptability.
Needle innovations, including the use of a shorter 8 mm needle, offer drugs, to enable the delivery of larger, more viscous drugs, without compromising end-user experience.16
IM RISK, INJECTION-RELATED ANXIETY & PAIN PERCEPTION
Standard needles for chronic disease treatment and parenteral administration are historically 12.7 mm (half an inch) long. Regarding the thickness of the different skin layers10, a 12.7 mm needle may reach the IM layer during a subcutaneous (SC) injection. This risk is increased in the absence of skin pinch (usually recommended with this needle length) or when done incorrectly and especially for populations with thin SC fat, such as people with low body mass index (BMI), children, and those with certain diseases.
Reducing the needle length to 8 mm would reduce the risk of accidental IM injection in most cases (Figure 1a) without increasing the risk of accidental intradermal (ID) injection (which may lead to unwanted immune responses12).
One therapeutic area that uses short needles for SC administration is endocrinology, mainly in the treatment of diabetes. Optimising insulin administration for patients while minimising the risk of IM administration, which can have severe health consequences, has been a key driver for new delivery solutions. Shorter needles are now commonly used by diabetics and enable efficient delivery of insulin into the SC space as recommended by the Forum for Injection Technique and Therapy: Expert Recommendations (FITTER).13
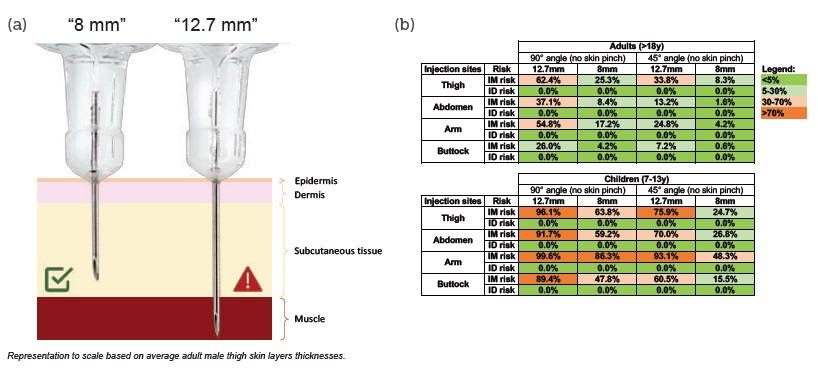
Figure 1: (a) Short needle (8mm) reduces IM injection risk without increasing ID injection risk. (b) IM and ID injection risk percentages per needle length, age, needle insertion angle (no skin pinch) and injection sites. Risks were calculated with an internal mathematical model18 based on human skin layers thicknesses (388 adults and 49 children 7-13 years).10-11
Concentrated biologics such as mAbs and other common therapeutics for other chronic conditions have their own pharmacokinetics/pharmacodynamics and physiological interactions, meaning the consequences of IM and ID injections should be carefully evaluated on a case-by-case basis when selecting the most appropriate drug delivery system.
Adopting a shorter 8 mm needle length reduces the risk of IM injection without increasing the risk of ID injection in both adults and children10,11 (see Figure 1b). On average, when using 8 mm needles, the risk of IM injection without skin pinch and at a 90° angle was reduced by 72% in adults and 32% in children (aged 7-13 years). The risk of IM injection in the thigh or abdomen – commonly used self-injection sites – was reduced by 59% and 77%, respectively, in adult patients; and by 34% and 35%, respectively, in children.
In addition to the benefit of reducing IM injection risk, shorter needles have a direct impact on injection-related anxiety and pain perception in patients using pens with exposed needles14. Moreover, needle length is one of the factors contributing to injection performance and flow.
INJECTION PERFORMANCE
When working with highly concentrated biologics, the viscosity and volume are key factors that directly impact several injection parameters such as the force and the duration of injection.
During manual injection, a higher drug viscosity would require a higher injection force to deliver the solution in the same amount of time. Mathematical simulations15 of injection forces were performed with both 12.7 mm and 8 mm needles, using different fluid viscosities at a given injection time of 15 seconds. The results showed that with low viscosity (1 cP), injection force remained essentially the same regardless of needle configuration. However, with higher viscosity (10 cP), the short 8 mm needle configuration led to a reduction of ~14% in injection force (Figure 2).
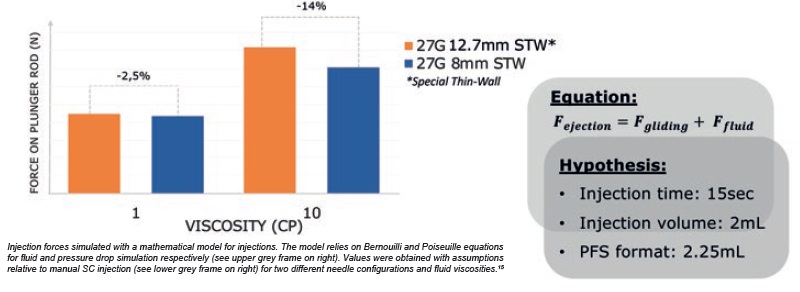
Figure 2: Injection force required to push on plunger rod is reduced with 8 mm needle.
A human factors validation study investigated the impact of injection force reduction by measuring end-user acceptance of simulated injections performed with 12.7 mm and 8 mm needles at different viscosities.16 Results showed that end-users positively felt the injection force reduction with the shorter 8 mm needle compared with the 12.7 mm needle for the 10 cp solution. That is to say, 40% of end-users found the injection force acceptable when using an 8 mm needle versus 15% when using a 12.7 mm needle (Figure 3).
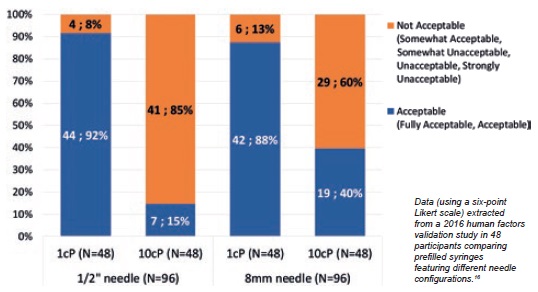
Figure 3: End-user injection force acceptance is enhanced with 8mm needle.
To address the specific needs of pharma companies developing viscous/ high volume (2.25 mL) biologics, BD has launched and industrialised a short 8 mm needle solution, based on its state-of-the-art BD Neopak™ glass prefillable syringe (Figure 4).
Figure 4: BD Neopak™ 2.25 mL glass prefillable syringes with 12.7 mm and 8 mm needles.
INNOVATIVE NEEDLE TECHNOLOGIES PUSH VISCOSITY BOUNDARIES
BD has a strong track-record in needle innovation, answering to specific and evolving needs of pharma companies and end-users. In the last decade BD worked to reduce needle insertion pain, with the launch of BD Physiolis™ 29G Thin Wall needle17, and to increase injection flow rate with BD Hyflow™ 27G special Thin Wall needle.
With manual delivery needle standards, pharma companies developing viscous drugs for chronic use may need to compromise on patient injection experience. One way to improve the performance of viscous drug delivery without increasing injection force is to increase the inner diameter of the needle.
“This innovation combining short 8 mm with ultra-thin wall technology therefore reduces the injection force, especially for viscous solutions, without compromising on needle insertion pain and pain-perception.”
To address this, BD is developing a prefilled syringe solution combining shorter needle length (8 mm) with ultra-thin wall (UTW) needle technology. This technology increases the inner diameter of the needle by reducing the thickness of the needle wall without having to increase the external diameter (see Figure 5). The outer diameter of a needle is known to be an important feature as it influences needle insertion pain perception and the level of injection-related anxiety experienced by patients.14,17
While at low viscosity (1 cP), injection force remains similar for all types of needle, mathematical simulations show that using an UTW needle can reduce injection force by 46% for highly viscous solutions 30 cP (Figure 5).
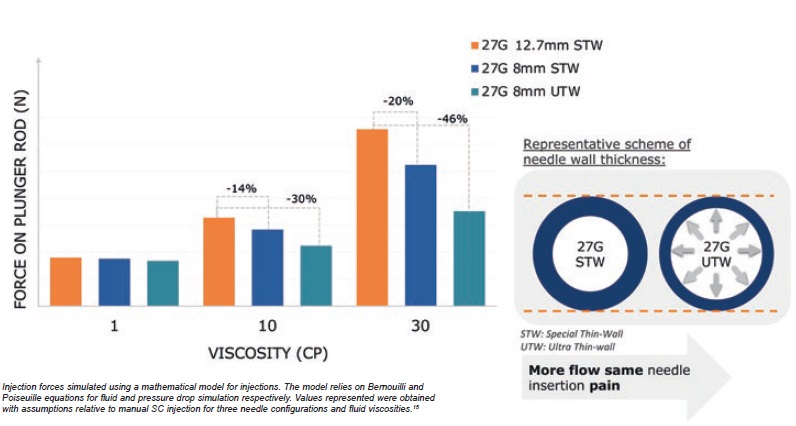
Figure 5: Combination of short 8 mm and thinner needle wall technology contributes to reduced injection force.
This innovation combining short 8 mm with UTW technology therefore reduces the injection force, especially for viscous solutions, without compromising on needle insertion pain and pain-perception.
INTEGRATED SYSTEM SOLUTIONS
With the aim of improving the self-injection experience for chronic disease patients in the home setting, pharma companies often develop self-injection devices combining a primary container (e.g. PFS) with needle-stick safety guard or autoinjector solutions (i.e. integrated systems). System integration challenges might arise due to the number of functional interfaces and especially when the different components are purchased from multiple vendors, as they must operate perfectly together once assembled.
To design a robust, high-quality device that will deliver the drug to patients safely and effectively with reproducible performance across millions of units, manufacturers need to ensure excellent compatibility between biologics, primary container and secondary device.
Developing and launching a drug-device combination product is a long and expensive journey for pharma companies and their partners. In order to ensure that pharma companies succeed in this process, BD, with its long history and experience in combination products, is well positioned to support the development of more robust, better-designed systems. BD is able to manage all the requirements through the cascading process from delivery system requirement definitions, sub-system requirements, component requirements, manufacturing process requirements during the definition and development phases. It covers all design control aspects including usability (human factors) engineering, preclinical and clinical evaluation.
Ideally positioned with its exhaustive portfolio of drug delivery solutions for the chronic market, BD offers seamless integration of short 8 mm needle BD Neopak™ 2.25 mL syringes with BD injection devices including the BD Intevia™ 2.25 mL large-volume autoinjector.
CONCLUSION
Shorter 8 mm needle solutions demonstrated several benefits versus the current 12.7 mm standard needle. Notably, occurrences of unwanted IM injections are reduced and better performance parameters such as injection force reduction for viscous solutions can be positively perceived by end-users. Furthermore, patients experienced reduction in both anxiety and pain perception when using shorter needles, improving their overall injection experience.
BD’s application of UTW technology to a shorter 8 mm needle should improve the end-user injection experience, taking it to the next level, by presenting the chronic disease market with a meaningful, viable solution that addresses the challenges related to high volume/highly viscous drugs that it faces.
Identifying enhanced needle solutions and offering integrated system solutions to serve chronic market unmet needs is a great step towards improved injection experience and quality of life for patients suffering from chronic diseases. BD adopts a patient centric design approach and develops drug delivery solutions tailored to home care, with the vision to improve patient adherence to chronic disease treatments.
REFERENCES
- Mathers C, Loncar D, “Projection of mortality and causes of death, 2016-2060”. PLoS Medicine, 2006, Vol 3(11), e442.
- “Chronic diseases and conditions are on the rise”. Company Web Page, PwC, Sep 2019.
- “World Preview 2018, Outlook to 2024 – 11th Edition”. Research Report, Evaluate Pharma, June 2018.
- “Market analysis and projections for chronic conditions”. Internal Analysis, BD, 2019.
- Kararli T et al, “Injectables, the New Oral?”. Contract Pharma Online, May 4, 2017.
- “Prefilled Syringe Customer Requirements Voice of the Customer Research”. Internal Study, “Willow”, BD, 2018.
- Shire S, “Strategies to deal with challenges of developing high-concentration subcutaneous (SC) formulations for monoclonal antibodies(mAbs)”. Monoclonal Antibodies (book), Chapter 7, pp 139-152.
- Yang Y et al, “Multi‐criteria manufacturability indices for ranking high‐concentration monoclonal antibody formulations”. Biotechnol Bioeng, 2017, Vol 114(9), pp 2043-2056.
- Neeraj JA et al, “Computational tool for the early screening of monoclonal antibodies for their viscosities”. MAbs, 2016, Vol 8(1), pp43-48.
- Gibney M et al, “Skin and subcutaneous adipose layer thickness in adults with diabetes at sites used for insulin injections: implications for needle length recommendations”. Curr Med Res Opin, 2010, Vol 26(6), pp 1519-1530.
- Lo Presti D, Ingegnosi C, Strauss K, “Skin and subcutaneous thickness at injecting sites in children with diabetes: ultrasound findings and recommendations for giving injection”. Pediatr Diabetes, 2012, Vol 13(7), pp 525-533.
- Baker M, Reynolds H, Lumicisi B, Bryson C, “Immunogenicity of protein therapeutics: The key causes, consequences and challenges”. Self Nonself, 2010, Vol 1(4), pp 314-322.
- Frid A et al, “New insulin delivery recommendations”. Mayo Clin Proc, 2016 Vol 91(9), pp 1231-1255.
- Schwartz S et al, “A multicenter, open-label, randomized, two-period crossover trial comparing glycemic control, satisfaction and preferences achieved with a 31 gauge x 6 mm needle versus a 29 gauge x 12.7 mm needle in obese patients with diabetes mellitus”. Clin Ther, 2004, Vol 26(10), pp 1663-1678.
- “Injection time and ejection force calculation”. Internal Study, BD, 2014.
- “Human factor validation study: Evaluation of BD Neopak™
- 2.25 mL in Non-Self-Injecting and Self-Injecting Populations.”
- Jaber A et al, “A novel needle for subcutaneous injection of interferon beta-1a: effect on pain in volunteers and satisfaction in patients with multiple sclerosis”. BMC Neurol, 2008; Vol 8, Article 38.
- Rini C et al, “Evaluating the Impact of Human Factors and Pen Needle Design on Insulin Pen Injection”. J Diabetes Sci Technol, Vol 13(3), pp 533-545.

