Citation: Sampathkumar K, Loo J, Kesavapany S, “Gastric Floating Microcapsules for the Management of Parkinson’s Disease”. ONdrugDelivery, Issue 122 (Jul 2021), pp 16–21.
Kaarunya Sampathkumar, Joachim Loo and Sashi Kesavapany discuss current treatments for Parkinson’s disease and introduce an extended-releasing technology for polypharmacy oral formulations.
“On average, LID affects 40% of PD patients after five years of treatment, and 90% of patients by 9–15 years of treatment.”
Parkinson’s disease (PD) is a degenerative disorder of the central nervous system and is the second most common neurological disorder after Alzheimer’s disease, with an estimated 10 million people being affected.1 The disease is characterised by an asymmetric onset of bradykinesia, resting tremor, rigidity and postural instability. Symptoms of this motor‐degenerative disease stem from the death of dopamine‐generating cells in the substantia nigra. The most common treatment for PD is with levodopa (LD), which can help to alleviate the symptoms of the disease by converting to dopamine in the brain. Levodopa improves disability and capacity to perform important activities of daily living. Approximately 85% of patients have some degree of benefit with this therapy.
ISSUES WITH LEVODOPA TREATMENT
LD is not without its issues, however, as chronic administration leads to a pharmacological problem – levodopa induced dyskinesia (LID). It is widely accepted that LID is due, at least in part, to the short half‐life of LD. Dyskinesia most commonly occurs at the time of peak LD plasma concentrations during intermittent or pulsatile LD stimulation – peak‐dose dyskinesia.2 Reviews of observational studies and clinical trials agree that, on average, LID affects 40% of PD patients after five years of treatment, and 90% of patients by 9–15 years of treatment.3 LID has been associated with exhaustion, fatigue and weight loss due to the excessive involuntary movement in patients, and it is said to limit the PD patient’s social life, causing feelings of isolation, frustration and depression.4
“A study on the direct healthcare costs and predictors of treatment costs associated with LID in the US found that the presence of LID resulted in an increase in total treatment costs of 29% and of PD related treatment costs of 78% when compared with costs incurred among those patients without LID.”
A study on the direct healthcare costs and predictors of treatment costs associated with LID in the US found that the presence of LID resulted in an increase in total treatment costs of 29% and in PD‐related treatment costs of 78% when compared with costs incurred among those patients without LID.5 Other factors influencing treatment costs were the use of other PD medications and the presence of select comorbidities (psychiatric, cardiovascular, chronic renal disease, injury and fracture). To mitigate LID, a continuous and non‐fluctuating provision of LD to the brain is therefore essential.
Current oral formulations of LD in the market fail to address the issue of fluctuating LD levels, whereby most patients are known to take up to five tablets a day,6 resulting in a rapid sinusoidal rise and decline of LD – the cause of LID. To provide a continuous delivery of LD, other pharmacological strategies, including intestinal infusion, administered through an external device, have since been explored.7 Intestinal infusion of LD, although effective, has been associated with procedural‐ and device related technical problems in 20% to 70% of patients.8 As oral tablets remain the simplest and most convenient form of drug administration, designing oral formulations that can provide a continuous delivery of LD to the brain is therefore the most feasible approach to mitigate LID in PD patients.
The innovation of an economical, convenient oral formulation that does not disrupt or alter the daily lives of PD patients, while mitigating LID, would therefore provide huge benefits to these patients, whether from a cost or quality‐of‐life perspective. Along these lines, LiberaTx’s technology aims to reduce the requirements and cost of current treatment.
THE TECHNOLOGY
“A preliminary pharmacokinetics study of LiberaTx’s patented extended-releasing polypharmacy oral PD formulations to deliver levodopa in mice has shown highly promising results.”
In view of the current clinical problems, the LiberaTx team sought to design a floatable, extended‐releasing formulation that permits the sustained release of polypharmacy (i.e. multiple drugs) to mitigate LID.
This strategy exploits the stomach as a drug reservoir for the slow release of levodopa into the upper intestinal tract, where levodopa is mainly absorbed by the body. This strategy prolongs the gastric retention time of the drugs, and allows for the drugs to be slowly absorbed in the upper gastrointestinal tract (Figure 1). Such a strategy is therefore similar to the levodopa intestinal infusion device but miniaturised into the form of an oral capsule. This avoids the technical problems and undesirable issues of the external infusion device, while avoiding the LID that comes with commercial oral PD tablets. Based on this, LiberaTx has patented a floatable microencapsulation technology that allows for the controlled, sustained release of multiple drugs (polypharmacy) from microcapsules, while avoiding drug-drug interactions.9,10
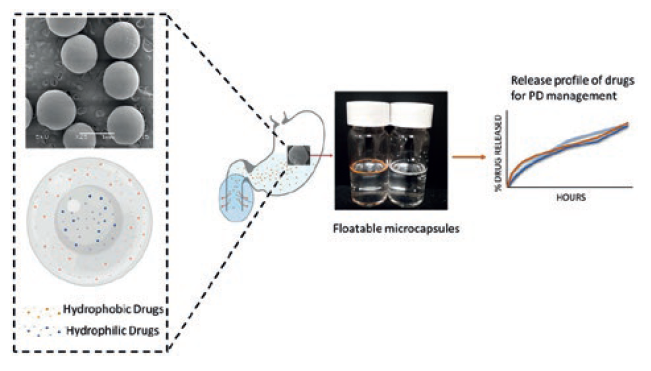
Figure 1: Graphic showing the floating microcapsule technology, drug localisation and release.
A preliminary pharmacokinetics study of LiberaTx’s patented extended‐release polypharmacy oral PD formulations to deliver LD in mice has shown highly promising results. If successful, this platform formulation, besides its intended use for PD, can also be exploited for sustained release of polypharmacy in the management of other chronic diseases where patients face a high pill burden.
A simple, economical, scalable and versatile encapsulation process was devised for the production of these multidrug loaded, sustained‐release gastric floating microcapsules.11,12 Microcapsules co‐loaded with LD, carbidopa (CD) and entacapone (ENT) were fabricated through a scalable emulsion technique. In order to attain prolonged gastric residence time, these capsules were designed to be hollow – i.e. of lower density – to attain better floating capabilities.
To achieve different drug release kinetics, several formulations were fabricated. For comparison purposes, an equal amount of PD drugs was encapsulated in these samples at a ratio of 4:1:8 (LD:CD:ENT), so as to replicate an equal drug ratio as commercially available PD tablets – i.e. Stalevo‐100 (Novartis, Basel, Switzerland).
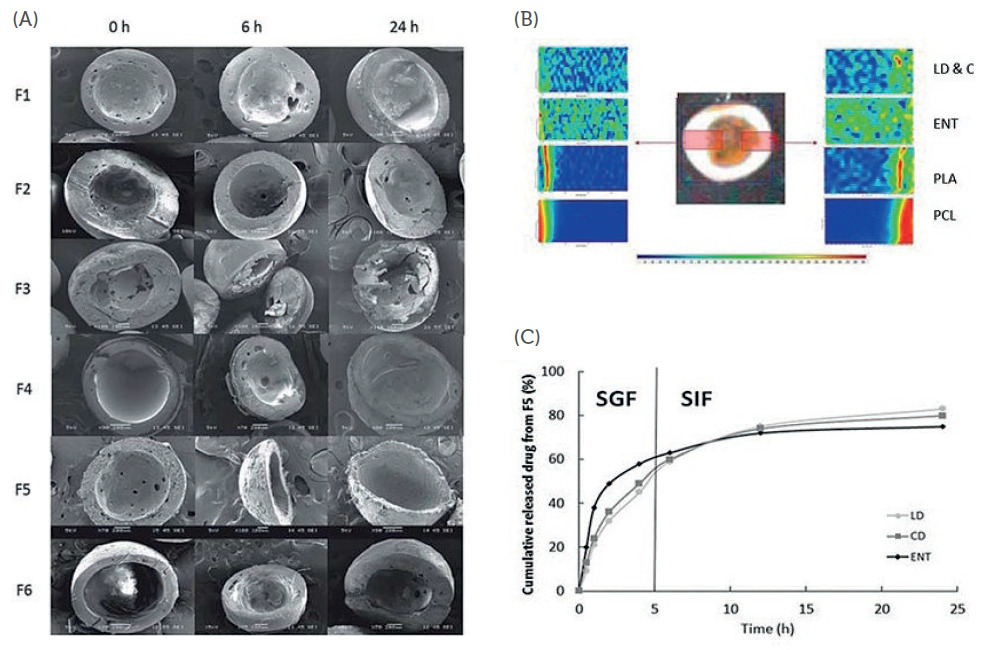
Figure 2: (A) Cross-sectional SEM images of different formulations of spray-coated microcapsules (SC-MC) loaded with LD, CD and ENT in SGF at different time points; (B) Raman mapping of a SC-MC showing the localisation of LD, CD and ENT in different compartments within the particle; (C) in vitro release profiles of LD, CD and ENT from optimised formulation in SGF and SIF (simulated gastric/intestinal fluids).
Figures 2A and 3A show the cross-sectional scanning electron microscopy (SEM) images of the formulations (from top to bottom) with increasing drug release time (left to right). Figures 2B and 3B show the localisation of the drugs in different compartments of the particle. To test the hypothesis that this advanced polypharmacy drug delivery system can achieve sustained and controlled release of three PD drugs, as opposed to commercially available tablets, the release profiles of the drugs were investigated.
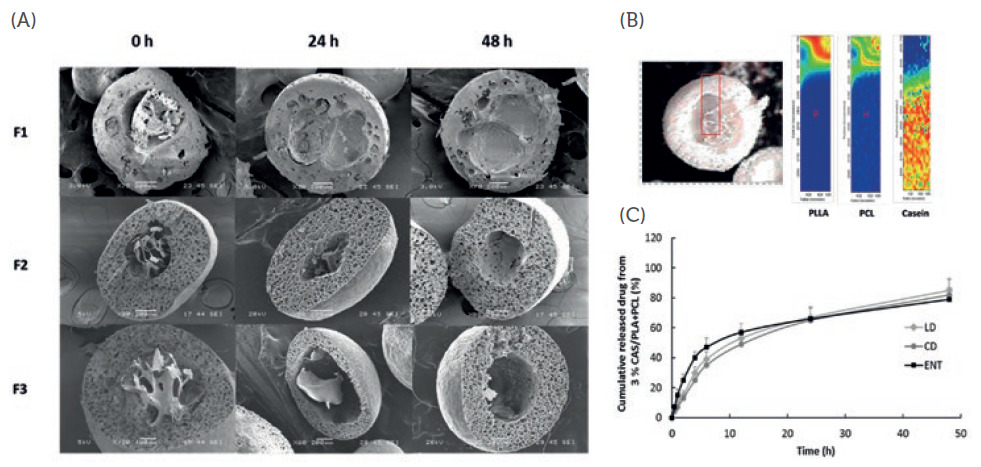
Figure 3: (A) Cross-sectional SEM images of different formulations of casein microcapsules (C-MC) loaded with LD, CD and ENT in SGF at different time points (B) Raman mapping of a C-MC showing the localisation of LD, CD and ENT in different compartments within the particle; (C) in vitro release profiles of LD, CD and ENT from optimised formulation in SGF and SIF (simulated gastric/intestinal fluids).
As shown in Figures 2(C) and 3(C), a sustained release of LD, CD and ENT was obtained from LiberaTx’s formulations. Pharmacokinetics studies of these formulations in mice showed promising results when compared with commercial formulations (i.e. Stalevo). LiberaTx’s extended‐release polypharmacy formulations (SC‐MC and C‐MC) have a superior absorption profile of LD compared with the control at the same drug dosages. Similarly, LiberaTx’s formulation showed better bioavailability of LD, and a raised and stable dopamine level in the brain (Figure 4).
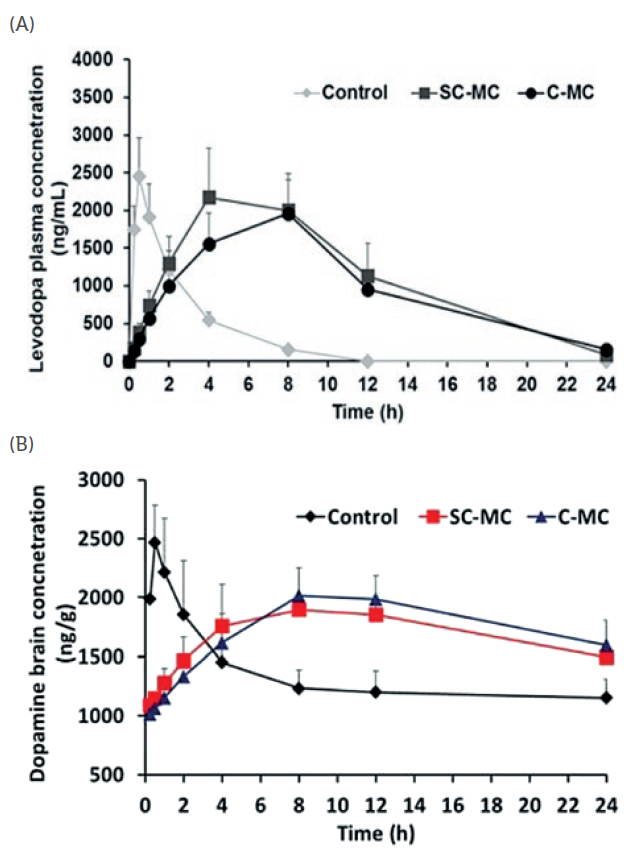
Figure 4: (A) Plasma concentrations of LD and (B) brain dopamine concentrations from control (i.e. commercial formulation) and LiberaTx’s two patented formulations (SC-MC and C-MC).
The floating microcapsules, as a platform technology, also aim to improve patient medication compliance by providing sustained release of polypharmacy for the management of chronic diseases. In a research paper by Health Prize Technologies and consulting company Capgemini, medication noncompliance was described as “one of the most serious problems in healthcare”, affecting all parties and causing more than just financial drawbacks.13 According to a recent report, medication non-adherence is costing the global pharmaceutical industry in excess of US$1 trillion (£720 billion) annually.14 This platform technology is expected to address this colossal issue by reducing the pill burden and improving patient medication compliance.
CURRENT MEDICATIONS AND THEIR LIMITATIONS
Currently there are several commercially available tablets for PD on the market – including Stalevo, Sinemet (Merck Sharp & Dohm, Kenilworth, US), Rytary (Impax Pharmaceuticals, Hayward, US) and Madopar‐HBS (Roche, Basel, Switzerland). LD is the main active ingredient in all these formulations. However, administering LD alone results in poor bioavailability.
To increase bioavailability, some formulations include other drugs, such as CD and/or ENT. For example, Sinemet (a two‐drug formulation) is a combination of CD and LD, and Stalevo (a three‐drug formulation) contains LD, CD and ENT. CD is an inhibitor of aromatic amino acid decarboxylation. ENT, a catechol‐O‐methyltransferase (COMT) inhibitor, is a nitro‐catechol‐structured compound. These drugs work synergistically to increase bioavailability of LD in the brain for conversion to dopamine. Table 1 summarises these marketed tablets and the issues associated with them. While there have been a number of extended-release formulations for LD/CD combinations, there have not been any such products for the LD/CD/ENT combination, until LiberaTx’s formulation.
| Marketed Technology | Issues with Marketed Tablets |
| Sinemet (Merck Sharp & Dohme) and Stalevo (Novartis) |
• Lack the capabilities of providing controlled and sustained release of multiple drugs. • Do not possess prolonged gastric retention time. • Although controlled release versions of these are available, their pharmacokinetics are still less than desirable. • Require multiple dosing daily, thus reduce patient compliance and cause LID. |
| Madopar‐HBS (Roche) |
• A levodopa and benserazide releasing floating system, which is used in PD. The controlled release is based on the dissipation of hydrated boundary layers upon the dissolution of gelatin capsules. • A single‐unit floating system, which is unreliable in prolonging the gastric retention time owing to its “all‐or‐nothing” emptying process. • Results in high variability in bioavailability and local irritation due to a large amount of drug delivered at a particular site of GI tract. • Although Madopar‐HBS showed improvement over standard Madopar, the bioavailability is reduced as compared with standard Madopar (60–70%), due to incomplete absorption. |
Table 1: Issues with current marketed tablets for Parkinson’s disease.
A secondary clinical problem that presents in PD patients is medication non‐compliance. Adherence to medication is critical for the management of chronic conditions. In fact, for neurologically incapacitated patients (i.e. stroke, Alzheimer’s disease, PD, mental disorders, etc.), poor adherence to prescribed drugs remains the key reason for sub‐optimal clinical outcomes. Medication non‐adherence is observed among 50% of patients with chronic illnesses, and the common reasons are forgetfulness and complex medication regimens,15 and is one of the most common causes of therapeutic failure, costing $290 billion per year in the US alone.16 Extended‐releasing polypharmacy oral formulations therefore address the issue of medication non‐compliance by lowering the pill burden, while enhancing recovery or managing chronic diseases.
REFERENCES
- “How Common Is Parkinson’s Disease?”. ParkinsonsDisease.net, Sept 2019.
- Ren T et al, “Sustained-release formulation of levodopa methyl ester/benserazide for prolonged suppressing dyskinesia expression in 6-OHDA-leisoned rats.” Neuroscience Letters 2011, Vol 502(2), pp 117–122.
- Coelho M, Ferreira J, “Epidemiology of Levodopa-Induced Dyskinesia.” Levodopa-Induced Dyskinesia in Parkinson’s Disease, 2014, pp 33–50.
- Thanvi B, Lo N, Robinson T, “Levodopa-induced dyskinesia in Parkinson’s disease: clinical features, pathogenesis, prevention and treatment.” Postgrad Med J, 2007, Vol 83 (980), pp 384–388.
- Suh D, Pahwa R, Mallya U, “Treatment patterns and associated costs with Parkinson’s disease levodopa induced dyskinesia.” J Neurol Sci, 2012, Vol 319(1-2), pp 24–31.
- Sethi K et al, “Levodopa/carbidopa/entacapone 200/50/200 mg (Stalevo® 200) in the treatment of Parkinson’s disease: a case series.” Cases Journal, 2009, Vol 2(1), pp 1–5.
- Schaeffer E, Pilotto A, Berg D, “Pharmacological strategies for the management of levodopa-induced dyskinesia in patients with Parkinson’s disease.” CNS Drugs, 2014, Vol 28(12), pp 1155–1184.
- Abbruzzese G et al, “Continuous intestinal infusion of levodopa/carbidopa in advanced Parkinson’s disease: efficacy, safety and patient selection.” Funct Neurol, 2012, Vol 27(3), p 147.
- Loo S, Lee, W, Wee P, “Floating capsules encapsulating particles loaded with one or more drugs.” Google Patents, 2016.
- Loo S, Jongsuep B, “Floatable pharmaceutical microcapsule composition.” Google Patents, 2020.
- Lee W et al, “Gastric-floating microcapsules provide controlled and sustained release of multiple cardiovascular drugs.” J Mater Chem B, 2013, Vol 1(8), pp 1090–1095.
- Baek J et al, “Multi‐Drug‐Loaded Microcapsules with Controlled Release for Management of Parkinson’s Disease.” Small, 2016, Vol 12 (27), pp 3712–3722.
- Mack H, “Pharma loses $637B annually due to medication nonadherence.” MobiHealthNews, 2016.
- Rao S, “How Medication Non-Adherence Impacts Brand Management.” https://www.pharmexec.com/view/5-signs-you-need-digital-healthcare-marketing-support (accessed 02 June).
- Lam W, Fresco P, “Medication adherence measures: an overview.” BioMed Res Int, 2015.
- Cutler R et al, “Economic impact of medication non-adherence by disease groups: a systematic review.” BMJ Open, 2018, Vol 8(1), e016982.

