To Issue 128
Citation: Beck R, Monchoix H, “Trials Without Tribulations: a Patient-Centric, Connected Injection Device for Decentralised and Hybrid Clinical Trials”. ONdrugDelivery, Issue 128 (Dec 2021), pp 40–43.
Rebecca Beck and Hervé Monchoix discuss how stakeholder needs are driving the development of a new, connected injection device from BD, to support decentralised and hybrid clinical trials.
“The adversity and challenges of the pandemic have catalysed movement towards decentralised clinical trials and their potential to overcome many of the restrictions imposed by covid-19.”
The challenges of clinical trials are well documented. These include growing trial costs and complexity,1 increased cycle time to database lock,2 non-adherence to protocol3 and data quality issues due to labour-intensive data transcription and complex integration processes.4 Clinical trials also face mounting challenges in recruiting and retaining study participants,5–7 in part due to the travel and time burdens placed on participants required to get to and from the trial site.
These issues were being addressed prior to the advent of the covid-19 pandemic. However, the adversity and challenges of the pandemic have catalysed movement towards decentralised clinical trials (DCTs) and their potential to overcome many of the restrictions imposed by covid-19.8 Indeed, the impact of the current pandemic on clinical trials has been significant – more than 1,200 clinical trials worldwide were disrupted due to covid-19.9 Significant decreases in new patients entering trials were observed globally, with decreases as severe as 87–98% in the most affected regions in April 2020, relative to figures from April 2019.9 However new patient recruitment improved thereafter.10 The covid-19 pandemic is considered to have greatly accelerated the move towards DCTs or hybrid decentralised/in-person clinical trials, with many of these gains expected to remain once the pandemic recedes.8
While the trend towards DCTs brings many advantages in terms of flexibility for participants, it does heighten the need for high-quality remote data collection – in particular, data related to the timing of drug administration.
To support the successful transition to decentralised clinical trials, BD is developing a new, connected injection device designed to meet the needs of each stakeholder group (participants, trial sponsors and contract research organisations (CROs)) undertaking DCTs or hybrid clinical trials.
“To inform the development and component definition of a new, connected device for the clinical market, and to increase confidence in stakeholder acceptance, BD undertook both qualitative and quantitative research, including industrial design and field research.”
DEVELOPING A CONNECTED INJECTION DEVICE FOR CLINICAL TRIALS
BD is a world leader in injection devices, with billions of products produced each year. Working on connected injection solutions is part of a larger focus at BD on patient-centric solutions. With this in mind, BD is presently developing a connected product capable of capturing and transmitting injection-related data. The device will be an optional upgrade of the BD Ultrasafe Plus™ Passive Needle Guard subcutaneous injection device, and it is intended to support clinical trials. The product under development intends to address the needs of patients, CROs and study sponsors for a safe, easy-to-use self-injection device that automatically captures reliable, high-quality and time-stamped data about the injection event from the patient (who may be self-injecting remotely) to the trial’s selected electronic platforms.
To inform the development and component definition of a new, connected device for the clinical market, and to increase confidence in stakeholder acceptance, BD undertook both qualitative and quantitative research, including industrial design and field research. This research is the result of collaboration between teams at BD, including BD Medical – Pharmaceutical Systems (BDM-PS) and BD Technology and Innovation (BDTI). The goals of this research were divided into the following areas:
- Product Journey – Establish the typical clinical product journey relative to the workflow of selected clinical stakeholders (researchers and patients).
- Concept Acceptance – Determine stakeholder acceptance of two conceptual workflow impacts.
- Preferred Workflow – Determine stakeholder preference of a conceptual connected workflow.
The result of the research-informed design is a connected injection solution comprised of four components: a Bluetooth-enabled injector, a data gateway, a BD Cloud solution application programming interface (API) and access to third-party platforms used for clinical trial data management (Figure 1).
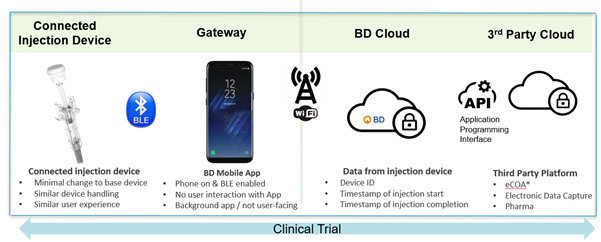
Figure 1: Contours of the BD Connected Solution. Informed by the research output, BD developed the solution architecture to match the needs of key stakeholders involved in clinical trials. eCOA: electronic Clinical Outcome Assessment tool.
CONTOURS OF THE BD CONNECTED SOLUTION
Connected Injection Device
The connected device will be available as an optional upgrade of the BD UltraSafe Plus™ Passive Needle Guard subcutaneous injection device. Wireless connectivity (Bluetooth Low Energy (BLE)) is assured by a module that is integrated into the plunger rod. This module provides the device ID and injection start and stop event capture. The BD connected plunger rod does not modify device operation and closely aligns with the form factor and ergonomics of the base device. The device requires minimal user intervention and incorporates a reliable, switch-based event capture mechanism. Injection-related data are transmitted directly to a background app on a smartphone, which transfers the data to the BD cloud. Depending on the application, these data can then flow via the gateway device (smartphone, tablet, etc) to the BD cloud and on to another relevant platform used by pharma or CROs.
BD Mobile Application
The app functions on a Bluetooth-enabled mobile device, typically a smartphone or smartphone-like device. The mobile app is being developed to serve as a gateway between the device and the cloud and to timestamp injection-related events received from the connected injection device. It will operate as a background app and require no user interaction (other than to ensure the phone is powered on and in range and BLE is enabled) and has no user-facing screens. It therefore will relieve the user from any additional steps.
BD Cloud
The BD cloud database will automatically receive the uploaded data from the app/mobile device. Data include the device ID, a timestamp of injection start and a timestamp of injection completion.
Data Collection and Third-Party Platforms
The three data points will flow from the device to the background mobile app and on to the cloud-based database. From there, the data can transfer to a third-party platform, be it an electronic clinical outcome assessment (eCOA) tool, an electronic data capture system or directly to a pharma platform, via APIs.
“BD is currently undertaking a human factors assessment to evaluate the usability and functionality of the connected solution. Outputs from this study will support and inform future development.”
DESIGN INFORMED BY RESEARCH
BD conducted both quantitative and qualitative research studies.3,11 The quantitative study comprised an online, double-blind survey with responses from 43 professionals working in the pharmaceutical and biotech industries, as well as those in CROs and eCOA providers. The qualitative study comprised one-on-one, in-depth interviews with clinical researchers and patients with experience in clinical trials involving subcutaneous drug delivery. These interviews were conducted via video link and lasted about 90 minutes each. Six clinical researchers and four patients were interviewed.
Study participants perceived several benefits of the proposed connected injection device solution for clinical trials, such as the elimination of manual data entry, avoidance of transcription errors, enhanced data quality and automatic data transfer. However, timely detection of deviation from protocol was perceived to be the most valuable benefit, addressing the biggest pain point recorded by respondents.
Study participants were asked to identify the most important data to be captured. The start time of the injection, device ID and the time of injection completion were cited as the most important data to be captured by a connected injection device within the context of clinical trials. Concerning device handling, participants emphasised that the connected solution should not bring with it any burdensome additional steps, that the form factor and function of the connected solution should closely align with that of the base device, and that the user should not be required to handle or interact with the phone at the time of injection.
PLUNGER ACTIVATION FORCE EQUIVALENCY
BD undertook a series of lab-based tests12 to confirm that the connected solution was fully compatible with the base device. Preliminary results of the mechanical testing indicate comparable performance of the BD Ultrasafe Plus™ Passive Needle Guard with the connected and standard plunger rods. The evaluation confirmed complete dose delivery, successful triggering of the safety mechanism and equivalence in plunger activation force. The success of event capture and data transfer were also tested and found to be functioning as expected (Figure 2).
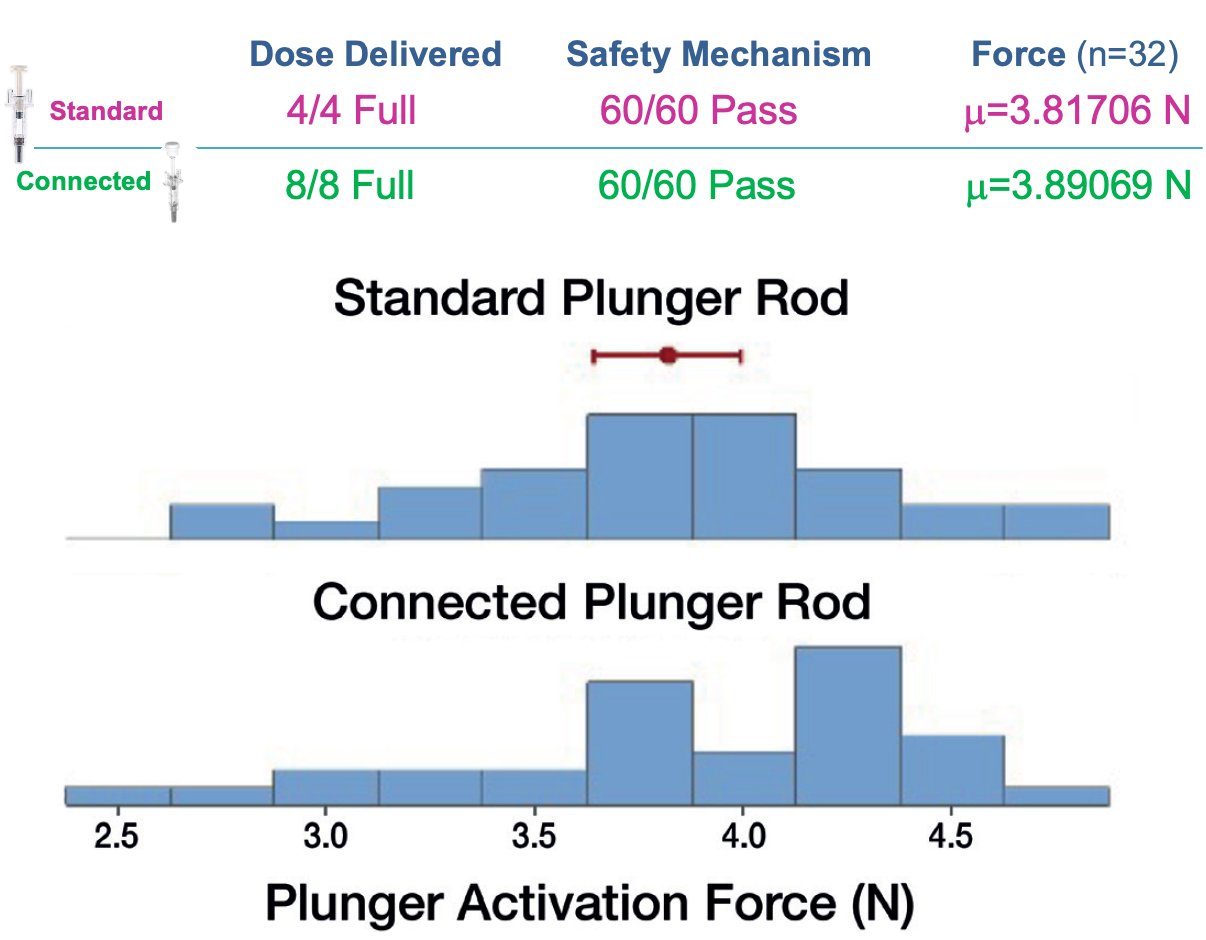
Figure 2: Preliminary results of mechanical testing indicate comparable performance of the BD Ultrasafe Plus™ Passive Needle Guard with standard (top) and connected (bottom) plunger rods.12
HUMAN FACTORS EVALUATION
BD is currently undertaking a human factors assessment to evaluate the usability and functionality of the connected solution. Outputs from this study will support and inform future development. The study includes both on-site and home-based simulated injections. This proof-of-concept study will provide an opportunity to gather detailed observations and user feedback on the handling and use of the device, and to assess the automatically captured injection data.
FINDINGS OF BD RESEARCH ALIGN WITH A LARGE, INDUSTRY STUDY
In a study undertaken by Informa Pharma Intelligence (London, UK) on behalf of Oracle Health Sciences (Austin, TX, US),13 participants were asked to identify what they consider to be the main challenges in adopting decentralised trials in their organisations. A total of 252 professionals involved in clinical trials at biopharmaceutical companies, medical device companies and CROs responded to the online survey. The top three challenges identified by the study were:
- Patient monitoring and engagement
- Ensuring data reliability and quality
- Data collection.
The connected injection device solution that BD proposes would address these fundamental challenges identified in this independent study. By providing reliable, automatic and real-time data capture, clinical researchers could remotely monitor compliance and engagement, and quickly detect and respond to any deviation from protocol. This valuable and timely insight into patient behaviour could be employed to strengthen engagement. The challenge of collecting high quality drug-administration data would also be addressed as the injection-related data would automatically be captured, time-stamped and securely transferred and stored.
LOOKING AHEAD
BD is actively responding to the challenges and needs surrounding connected solutions for injection devices, and is working to facilitate digitally enabled clinical trials. BD is presently identifying partnerships with pharma to run pilot studies.
REFERENCES
- Getz KA, Campo RA, “Trial watch: Trends in clinical trial design complexity”. Nat Rev Drug Discov, 2017, Vol 16(5), p 307.
- “Industry Survey Reveals Clinical Data Management Delays Are Slowing Trial Completion”. PDF, Veeva, Sep 21, 2017.
- Delmas I, “Final Report: Value assessment of a subcutaneous connected device”. Internal Study, 2020, GLG Projects.
- Kondo H et al, “Evaluation of Data Errors and Monitoring Activities in a Trial in Japan Using a Risk-Based Approach Including Central Monitoring and Site Risk Assessment”. Ther Innov Regul Sci, 2021, Vol 55, pp 841–849.
- Chaudhari N et al, “Recruitment and retention of the participants in clinical trials: Challenges and solutions”, Perspect Clin Res, 2020, Vol 11(2), pp 64–69.
- Ross S et al, (1999), “Barriers to Participation in Randomised Controlled Trials: A Systematic Review”, J Clin Epidemiol, 1999, Vol 52(12), pp 1143–1156.
- Tudur Smith C et al, “The Trials Methodological Research Agenda: Results from a Priority Setting Exercise”, Trials, 2014, Vol 15, Article 32.
- “No place like home? Stepping up the decentralization of clinical trials”. McKinsey & Company, June 10, 2021.
- “Covid-19 Pandemic Disrupted More Than 1,200 Clinical Trials: Global Data”, Pharmaceutical Technology, March 2, 2021. Accessed August 23, 2021.
- “COVID-19 and Clinical Trials – The Medidata Perspective – Release 9.0”. PDF, Medidata, September 21, 2020.
- “Connected Syringe – Research Report”. Internal study, 2020, BD.
- Internal BD lab report.
- “The Accelerated Evolution of Clinical Trials in a Pandemic Environment”. Research Report by Pharma Intelligence Informa, on behalf of Oracle Health Sciences, Nov, 2020.

