To Issue 130
Citation: Patel H, Holmes C, Wilson B, “Sustained, Controlled Drug Delivery to the Eye”. ONdrugDelivery, Issue 130 (Mar 2022), pp 28–30.
Harsh Patel, Cyonna Holmes and Brian Wilson consider the challenges and advances in sustained-release ocular drug delivery.
“Improving efficacy and providing sustained delivery options for small molecules, biologics and RNA are critical for improving treatment options for chronic eye conditions.”
Chronic eye diseases are the primary drivers of blindness and low vision, and the prevalence of these diseases is only expected to increase over the next five years due to an increased incidence of underlying conditions and the ageing population. Yet, there are still unmet needs in treating these diseases effectively. Innovative treatment approaches are needed to address the patient treatment burden and ineffective therapeutic delivery. As a result, improving efficacy and providing sustained delivery options for small molecules, biologics and RNA are critical for improving treatment options for chronic eye conditions. The ocular drug delivery market is expected to grow at a compound annual growth rate¹ of 6–9% as innovative approaches enter the market to address growing patient populations with glaucoma, chronic inflammation, diabetic retinopathy and macular degeneration.
CHALLENGES WITH CURRENT DOSE FORMS AND TRENDS TOWARDS SUSTAINED DELIVERY
Traditional topical formulations and intravitreal injections dominate the ophthalmic drug market. However, topical formulations have been associated with a low estimated patient adherence rate of 40–50%2 and poor bioavailability due to ocular barriers such as tear turnover, blinking and corneal and conjunctival barriers. Similarly, frequent intravitreal injections have negative side effects such as retinal toxicity, aggregation from injected materials, damage to the retina and lens, elevated intraocular pressure and inflammation due to repeated scleral puncturing.3 To overcome these challenges, sustained, controlled delivery of small molecules and large biologics is needed, and these approaches are gaining traction over current dose forms when treating chronic eye diseases.4 Successful application of such systems can significantly reduce the treatment burden to patients and, in turn, facilitate lower overall cost to the healthcare system.5
“An EVA implant in the suprachoroidal space serves as a unique approach to deliver therapeutic agents with various molecular weights.”
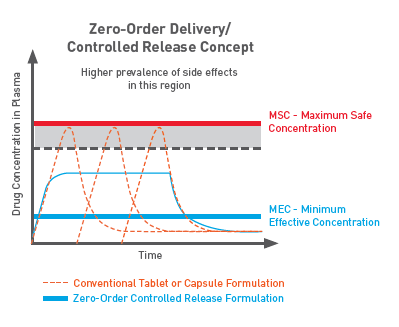
Figure 1: EVA is a biostable polymer offering better control of drug dosing, and eliminating undesirable bursts, with a zero-order controlled-release profile.
POLYMERIC IMPLANT FOR OCULAR DRUG DELIVERY
Polymeric implantable drug delivery systems remain a promising option for sustained, controlled delivery of therapeutics in the eye. The controlled, continuous release of therapeutic (Figure 1) from a polymeric implant removes the variability of patient compliance, improves bioavailability and may mitigate issues associated with repeated injections. Sustained drug release is achieved by the adjustable diffusion of the drug from such a system, which improves the half-life of the molecule, delays degradation and reduces elimination.
Polymeric approaches are being considered to leverage the versatility of biocompatible thermoplastics to:
- Provide tunable drug release of molecules ranging from small molecules to large biologics
- Provide innovative shapes and designs for customised implant delivery options
- Incorporate drug elution to existing medical devices to provide dual functionality.
“Biodurable ocular implants can be designed with high drug loading without compromising mechanical integrity or long-term release kinetics.”
BIODURABLE IMPLANT ADVANTAGES
Polymer-based, biodurable ocular implants offer distinct advantages in ocular delivery and have demonstrated versatility in the delivery of small molecules to large biologics. Biodurable ocular implants can be designed with high drug loading without compromising mechanical integrity or long-term release kinetics. High drug loading is critical in achieving drug-release profiles of six+ months that are relevant to the treatment of chronic eye diseases that use a combination of drugs or a high molecular weight API. Additionally, a reliable drug-release profile can be achieved with a biodurable implant as this approach does not rely on a bulk degradation mechanism with less predictable drug release. Biodurable implants also reduce the risk of drug molecule instability that may be caused by polymer degradants from erodible materials. As a result, release and accumulation of these degradants does not occur, thus mitigating inflammatory risk.
TUNABILITY FOR PRECISE DELIVERY
Biodurable implants using polymers such as ethylene-vinyl acetate (EVA) can achieve tailored release profiles over a wide range of molecules. Tunability can be further achieved through the pairing of drug-filled cores and customisable porous membranes. This approach adds another layer of adjustability as both the core and membrane properties can be modified to achieve a range of release profiles for small molecule, biologic and RNA delivery. Additionally, these two tunable components of an EVA implant – the core and membrane – also allow for dual release of more than one molecule through separate mechanisms, resulting in a fixed-dose combination implant.
TARGETED DELIVERY
Nearness of an implant to a targeted region can increase the therapeutic bioavailability of a molecule. As a result of localised biodurable implant delivery, lower drug concentrations are needed to elicit the desired therapeutic effect within the eye.
Due to the high compartmentalisation within the eye, unique locations for biodurable implant placement exist. As an example, suprachoroidal administration appears to be a promising and less-invasive technique for treatment of ocular posterior segment diseases. A polymeric implant must provide flexibility to mould into various shapes and sizes that may fit spaces such as the suprachoroidal space while maintaining mechanical integrity. As a result, an EVA implant in the suprachoroidal space serves as a unique approach to deliver therapeutic agents with various molecular weights.
Sustained delivery of molecules to treat chronic eye diseases can be achieved through polymeric implants, and biodurable implants offer an innovative approach to achieve desired therapeutic release profiles. As prevalence and patient needs continue to increase, ocular therapeutics need to adopt newer dosage forms to overcome challenges associated with traditional routes of administration. Biodurable implants address this growing unmet need and are ideal for the treatment of chronic eye disorders.
CELANESE SUSTAINED-RELEASE DRUG DELIVERY SOLUTIONS
Celanese has extensive expertise as a solutions provider for the pharmaceutical and medical device industry. Through collaboration with its customers, the company solves challenging drug delivery problems with innovative solutions that improve patient care. VitalDose® EVA, a pharmaceutical-grade polymer, is in pipeline and commercialised products and can be manufactured and designed to address a wide range of therapeutic areas and molecule types. VitalDose is a registered trademark of Celanese. Additionally, the Celanese Pharmaceutical Laboratory offers formulation support for early-stage pharmaceutical research and development.
REFERENCES
- “Ocular Drug Delivery System Market”. Fact.MR (2021), Research Report for Celanese.
- Tapply I, Broadway DC, “Improving Patient Adherence to Topical Medication in patients with Glaucoma”. Patient Prefer Adherence, 2021, 15, pp 1477–1489.
- Nayak K, Misra M, “A review on recent drug delivery systems for posterior segment of eye”. Biomed Pharmacother, 2018, 107, pp 1564–1582.
- Kim HM, Woo SJ, “Ocular Drug Delivery to the Retina: Current Innovations and Future Perspectives”. Pharmaceutics, 2021, Vol 13(1), p 108.
- Wittenborn JS et al, “The economic burden of vision loss and eye disorders among the United States Population Younger than 40 Years”. Ophthalmology, 2013, Vol 120(9), pp 1728–1735.

