To Issue 134
Citation: Marmot R, “How Digital Therapeutics Add Value to Treatments for Complex Conditions”. ONdrugDelivery, Issue 134 (Jun 2022), pp 22–25
Romain Marmot discusses how external partners with a track record of developing digital therapeutics for numerous chronic conditions and therapies can help life science organisations implement digital solutions across their pipeline, adding value to each therapy while improving medication adherence and clinical outcomes.
While the term “digital therapeutics” has only been widely used for the last few years, the concept of delivering treatment and prevention programmes through digital applications has been studied since the turn of the century, when researchers were calling them “Internet interventions”.
“For therapies that patients may take only once every few weeks, the consequences of a missed or incorrect dose can be significant.”
This means that the market for digital therapeutics is more mature than it may seem. The Digital Therapeutics Alliance product library lists nearly two dozen products that meet the organisation’s core principles. These include publication of peer-reviewed outcomes, clearance or certification by appropriate governing bodies, and collection and analysis of real-world evidence.
It is clear that digital therapeutics have established their value proposition for care delivery and patient outcomes. The challenge for today’s life science organisations is being able to deliver this digital experience at scale for the therapies they are bringing to market and for the various patient populations they are aiming to serve, all while remaining aligned with established provider workflows and standards of care.
HELPING PATIENTS MANAGE THE COMPLEXITY OF CHRONIC CONDITIONS
One important element of the value proposition for digital therapeutics is their potential to assist patients who must manage a chronic condition, such as diabetes, cancer or an autoimmune disease, throughout their lives. Lifelong management is possible today, largely due to the development of therapies that increasingly target the biological process, rather than simply treating the symptoms of the disease.
If there is a downside to highly targeted therapies, it is that they tend to require more engagement than a once-a-day pill. Inhalers for asthma or other respiratory conditions are most effective when patients shake the device and take a breath before actuating the inhaler. Injectable therapies for conditions ranging from diabetes to autoimmune diseases require a specific angle for properly dispensing each dose. Other medications come with dosing requirements that change over time.
“A digital solution can provide patients with a mix of educational materials, individualised dosing guidelines, self administration support and tools for tracking and managing potential side effects – all specifically created for their targeted therapy.”
This is a key unmet need in chronic condition management. Clinical teams can train patients at the point of care, but they typically cannot be present when patients administer a therapy at home. Additionally, dosing requirements and other aspects of self-administration often differ from one patient to another. It can be difficult for clinical teams to address the nuances of dosing, self-administration, actuation or angle of injection in a short clinical appointment. For therapies that patients may take only once every few weeks, the consequences of a missed or incorrect dose can be significant.
This is where digital therapeutics can play a pivotal role. Instead of receiving generic pamphlets about managing their chronic condition, a digital solution can provide patients with a mix of educational materials, individualised dosing guidelines, self-administration support and tools for tracking and managing potential side effects – all specifically created for their targeted therapy. Advanced solutions may even come with a training device to help patients better understand what it feels like to spray or inject the proper dose without needing to administer the therapy itself.
BEST WHEN ALIGNED WITH CLINICAL WORKFLOWS AND STANDARDS OF CARE
Through these features, digital therapeutics have the potential to increase the clinical effectiveness of a therapy, boost patient confidence in their ability to manage their condition and reduce the friction that clinical teams often face when patients have been prescribed a highly targeted therapy with a steep learning curve.
“By increasing the visibility of numerous aspects of a patient’s response to a given therapy, digital therapeutics give clinical care teams new insights into patient outcomes.”
That third benefit – reducing friction – is an important factor in the effective development, implementation and use of digital therapeutics. After all, a digital solution that does not align with existing standards of care or with existing clinical workflows will only add complexity to the care process, limiting adoption among prescribers and access to patients who would benefit from a digital solution. Let us consider each of these points in greater depth. First, there are standards of care for the management of chronic conditions already in place in hospitals, health systems and clinics around the world. These standards are based on a wide body of peer-reviewed clinical evidence and, through the experiences of frontline caregivers, have been uniquely tailored to meet the needs of local populations.
Rather than create a new standard of care, the most effective digital therapeutics elevate this existing standard of care. These solutions address the key challenges of chronic condition and medication management, while routinely collecting valuable data on metrics such as medication adherence, potential side effects, disease progression and changes in vital signs.
By increasing the visibility of numerous aspects of a patient’s response to a given therapy, digital therapeutics give clinical care teams new insights into patient outcomes. Providers can make more informed care decisions, proactively change care pathways instead of waiting for patients to come to them and take action without requiring an in-person visit, removing a barrier to care for many patients.
However, this can only happen if a digital solution aligns with existing clinical workflows. Integration with industry leading electronic health record (EHR) systems is a must-have. Requiring providers to leverage a separate clinical application with a unique username and password is a non-starter in any clinical setting.
Two additional factors contribute to successful alignment with clinical workflows, the first being where the digital therapeutic should integrate within the EHR. Research has shown that the average care appointment lasts 18 minutes – to make the most of this limited time, physicians need input and support from the digital solution at the moment when a conversation about therapeutic options is most likely to be taking place.
The second factor is how information should be presented within the EHR. As clinical teams are already inundated with data from numerous sources, any data coming from a digital solution needs to be presented contextually, focusing solely on what is actionable for the healthcare provider and what is relevant for the care decision that must be made at that time. This is true both at the point of care and as clinical teams review data between visits.
MAKING DIGITAL THERAPEUTICS DESIGN A PRODUCT DEVELOPMENT PRIORITY
While the benefits of digital therapeutics are clear, so too are the challenges of incorporating them into clinical workflows. The best way to meet these challenges is with a carefully planned and executed product design process that considers how a digital solution will be used alongside a given therapy (Figure 1).
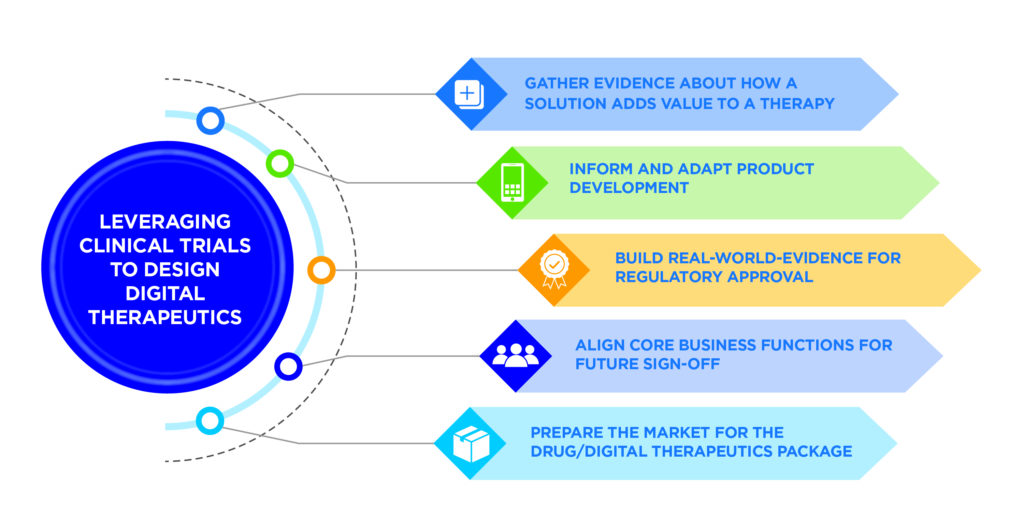
Figure 1: Making digital therapeutics design a product development priority.
This design process cannot be an afterthought. In order to bring value to patients in the real world, design of digital therapeutics should begin well before product launch, ideally in parallel with drug development. Starting the design process during clinical development offers five advantages:
- Just as the life science organisation generates a body of evidence about the safety and efficacy of the therapy being studied, the developer of the digital therapeutic gathers evidence about how a solution adds value to a therapy – a vital component of product approval.
- Feedback about how the digital therapeutic is used in conjunction with the therapy can inform numerous aspects of product development, such as integration into the clinical workflow or the patient-facing user experience. This allows developers to make early modifications and continue to test the solution on users (trial participants).
- Evidence gathered through the digital solution provides the life science organisation with a key source of patient-reported data, which generates additional evidence that can supplement an application for regulatory approval, as well as inform decisions about additional use cases for a given therapy.
- Conducting digital therapeutic design alongside the clinical development of the drug puts the digital solution in front of the core business functions that must ultimately sign off on it – legal, regulatory, clinical, compliance, quality assurance and, ultimately, marketing and commercial.
- The therapy and its accompanying digital therapeutic can come to market at the same time and be promoted as an all-in-one package. This clarity in messaging will shorten the learning curve for educating providers and patients about the digital solution, which will contribute to increased adoption.
“While the benefits of digital therapeutics are clear, so too are the challenges of incorporating them into clinical workflows.”
LEVERAGING THE EXPERIENCE OF PROVEN PARTNERS
Developing a digital therapeutic in parallel with the therapy it will support may make practical sense but many life science organisations struggle to do this, which is understandable. These organisations are steadfastly focused on gaining regulatory approval for the therapy under development, especially as a clinical trial reaches Phase III. In many cases, digital therapeutics are not a core competency for such organisations.
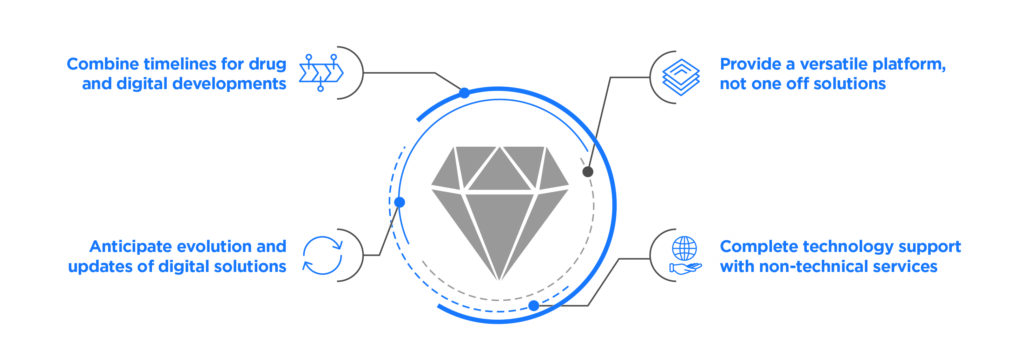
Figure 2: Leveraging the experience of proven partners.
For these organisations, the value of partnership with industry leaders becomes clear – particularly partners with a proven track record of supporting therapy assets throughout the product lifecycle and a decade or more post-market (Figure 2). There are four areas where partnership proves valuable:
Moving at the Right Pace
It may take a life science organisation 15 years to develop a molecule for the market. This is in stark contrast to the pace of developing digital solutions, which could go through a dozen iterations in the same length of time. Experienced partners can balance the slow pace of drug development with the fast pace of software development and manage product timelines accordingly.
Future-Proof Development
Likewise, while a drug rarely changes in formulation, a digital solution will change frequently, primarily as web browsers or mobile operating systems and devices are updated. The right digital therapeutics partner will be ahead of the curve here, building product components in such a way that they are minimally impacted by updates to consumer devices and therefore require little downtime to fix.
The Flexibility of a Platform
A digital therapeutic developed as a point solution will quickly outlive its usefulness if it cannot be applied to a different therapy’s unique challenges. The same is true of an underlying infrastructure that can only be accessed with significant custom development. Life science organisations should seek partners that offer a platform with a range of available services that can accommodate differences in clinical workflow, EHR integration, patient needs and hardware support. A broad spectrum of capabilities is better suited to meet the needs of multiple digital solutions.
Non-Technical Services
In addition to the platform and other technology infrastructure, a digital therapeutics partner should be able to provide non-technical support services. One example is market access, as regulations (as well as overall market characteristics) differ dramatically across different geographic areas. Another example is payer reimbursement, which can be significantly different for digital therapeutics than for therapies themselves.
CONCLUSION
In just a few years, digital therapeutics have evolved into valuable tools for helping patients manage chronic conditions. These digital solutions work best when they have been designed to complement a specific therapy, beginning from product development and extending into post-market support.
Many life science organisations lack the in-house expertise to build digital therapeutics on their own, especially amid the myriad challenges of bringing a therapy to market. External partners with a track record of developing digital therapeutics for numerous chronic conditions and therapies can help life science organisations implement digital solutions across their pipeline, adding value to each therapy while improving medication adherence and clinical outcomes.
For more information on Digital Therapeutics, visit: www.aptar.com/resources/how-digital-therapeutics-add-value-to-treatments-for-complex-conditions
Previous article
HOW DIGITAL UX IN CLINICAL TRIALS CAN IMPROVE MEASURESNext article
THE SMART INHALER REVOLUTION: ARE WE THERE YET?
