To Issue 137
Citation: Lai J-R, Cheng T-C, Li B, “A New MEMS Engine for Large-Volume Subcutaneous Injectors”. ONdrugDelivery, Issue 137 (Sep 2022), pp 36–41.
Jiunn-Ru Lai, Tsung-Chieh Cheng and Brian Li discuss how MicroMED’s Primary Actuator technology for driving high-volume, high-viscosity injection devices compares with current spring and motor technologies.
Lately, a significant trend has been noted in the biologics market – the shifting of the preferred administration route from intravenous (IV) towards subcutaneous (SC) injection.1 Due to the challenge represented by the frequent need for large-volume doses, SC delivery of biologics faces difficulties with solubility and large-volume form factors. This large-volume requirement is also difficult for traditional SC injection techniques due to the pain and tissue responses it causes. As such, there is a clear need for new delivery technologies to assist both patients and caregivers in improving the experience of large-volume injections.
Pharma companies have adopted two major approaches to resolve these large-volume SC injection challenges – manual SC injection formulation and device-assisted injection technology. For example, some blockbuster antibody biologics, including Herceptin® (trastuzumab, Genentech), Rituxan® (rituximab, Genentech) and Darzalex® daratumumab, Janssen), now have both IV and SC versions (specifically formulated for manual injection).2 On the other hand, advanced injection devices, such as autoinjector or on-body injectors (OBIs), have demonstrated huge potential to enable SC administration of biologics.
Many advanced injection devices have shown promising performances in both at-home and clinical settings. 2.25 mL autoinjectors are one of the large-volume solutions launched by many traditional 1 mL autoinjector manufacturers.3 Beyond 2.25 mL formats, even larger volume autoinjectors, including 5 mL and 10 mL,4,5 are on their way to the market. Meanwhile, in the world of OBIs, the 2 mL Neulasta Onpro for pegfilgrastim (Amgen)6 and the 3.5 mL Repatha Pushtronex for evolocumab (Amgen)7 have already received market approval and proven successful on the market. More OBIs, including some with volumes larger than 5 mL,3 are now on the way.
Springs and motors are commonly used to provide the driving force in large-volume injectors – however, they are not without their flaws compared with more modern technologies (Table 1). For example, a preloaded spring can generate an initial impact force capable of breaking a 1 mL glass prefilled syringe (PFS). This issue can be more serious when dealing with larger volume PFSs, such as 2.25 mL, 5 mL or 10 mL. Another example is the low driving force provided by micro motors, due to the fact that a motor’s torque is proportional to the radius of its rotor. Therefore, to generate a large torque force to drive an injection, a micro motor has to increase its rpm (rotational speed), resulting in high frequency noise and significant vibration during use, making it difficult to meet the requirements of advanced large-volume injectors.
| Large-Volume Engine Mechanism |
Spring | Micro-Motor | Primary Actuator |
| Initial Impact | ✶ | ✶✶✶ | ✶✶✶ |
| Maximum Driving Force | ✶✶ | ✶ | ✶✶✶ |
| Storage Load | ✶ | ✶✶✶ | ✶✶✶ |
| Stable Injection Flow | ✶ | ✶✶ | ✶✶✶ |
| Size of Device | ✶ | ✶✶✶ | ✶✶✶ |
| Noise | ✶✶ | ✶ | ✶✶✶ |
| Connectivity Integration | ✶ | ✶✶✶ | ✶✶✶ |
| Cost | ✶✶✶ | ✶ | ✶✶ |
Table 1: Comparison of the current driving technologies for large-volume injectors. Performance scale: ✶✶✶ Excellent, ✶✶ Good, ✶ Normal.
MICROMED’S PRIMARY ACTUATOR
Previously, MicroMED presented an innovative micro linear actuator – the Primary Actuator® (Figure 1 and Table 2). The company’s goal is to apply this small-footprint, accurate and powerful microelectromechanical system (MEMS) to solve the current unmet needs in large-volume SC drug delivery.8 This MEMS engine is an enabling driving force solution evolved from the semiconductor industry, offering superior strength and accuracy for injections, a broad range for injection rate and a low cost. These qualities make this technology particularly suitable to address the needs of large-volume, high-viscosity SC biologic injections.
| PA-001-6W | PA-001-12W | PA-001-24W | |
| Input Voltage | 6.0 V | 8.0 V | 12.0 V |
| Input Power | 6.0 W | 12.0 W | 24.0 W |
| Stroke | 28 mm | 40 mm | 40 mm |
| Maximum Static Force | 1000 N | 1000 N | 1000 N |
| Ingress Protection | >IP66 | ||
| Operating Noise | <25dB | ||
| Operating Temperature | 0°C – 60°C (32°F – 140°F) |
||
| Pin Length | 5.3 mm | 20 | 20 |
Materials:
|
Stainless Steel Polycarbonate Copper |
||
Dimension:
|
12 x 12 x 10.8 mm |
15 x 15 x 15.8 mm |
23 x 23 x 16.3 mm |
| Free Sample Available | No | No | Yes |
Table 2: Standard product specifications for the Primary Actuator. Customisation of the device is available upon request.

Figure 1: 3D model and photo of the MicroMED Primary Actuator.
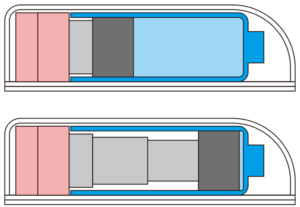
Figure 2: Telescopic rod actuation of the MicroMED Primary Actuator.
The device is specifically designed to drive the actuation of primary containers such as PFSs or prefilled cartridges with a telescopic push rod mechanism (Figure 2). This miniaturised engine uses a small but powerful MEMS microchip and integrated micro control circuitry to provide the injection force, speed and control required by even the most challenging high-viscosity, large-volume biologics.
A strong partnership between MicroMED and industry partners will enable the MEMS engine technology to be developed into a high quality commercial injector product that meets the true need of today’s large-volume biologic injectables. The following sections showcase the performance of the Primary Actuator in case studies where it was integrated into injector prototypes with the following form factors (Figure 3):
- A 1.0 mL autoinjector
- A 2.25 mL autoinjector
- A 5.0 mL large-volume autoinjector
- A 10 mL large-volume OBI.
PRIMARY ACTUATOR IN LARGE-VOLUME AUTOINJECTOR
Force Performance
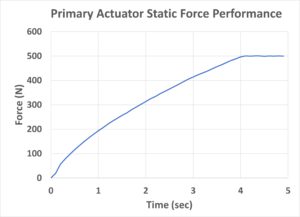
To begin with, both the static and dynamic force-to-time responses for the Primary Actuator were calibrated. First, the real-time static force output of the actuator was measured using a load cell force sensor (Figure 4). The peak driving force was over 500 N at a time of around 4 seconds. While higher forces were also tested (up to 1,000 N), the 500N force measured already surpasses most of the currently used driving technologies (springs and motors) by more than 10 times.
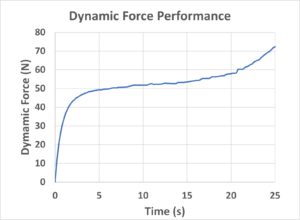
Second, the dynamic force output (Figure 5) of the Primary Actuator was measured in real time while driving a 5.0 mL PFS with a 26G needle and 8.5 cP testing fluid to simulate actual administration conditions. The force smoothly ramped up from zero until reaching a steady-state operating force of 50–60 N. When the plunger reached the end of the primary container (at around 20 seconds), the force increased linearly, showing a characteristic feature of the dynamic force performance of the Primary Actuator.
Flow Control Performance
The injection flow control for the Primary Actuator was assessed in real time as the driver for three different autoinjectors:
- A 1 mL PFS with 26G needle and 1 cP testing fluid (Figure 6)
- A 2.25mL PFS with 26G needle and 28 cP testing fluid (Figure 7)
- A 5 mL PFS with 26G needle and 28 cP fluid (Figure 8).
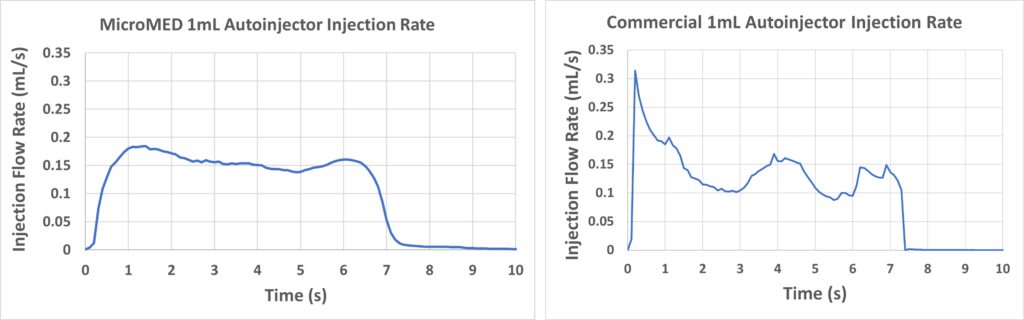
Figure 6: Injection rate performance of the Primary Actuator (left) and the off-the-shelf autoinjector (right) driving a 1 mL PFS.
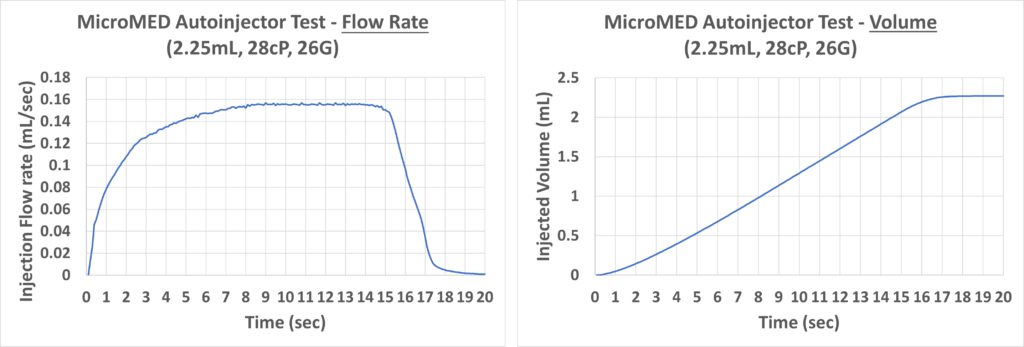
Figure 7: Injection rate (left) and injected volume (right) performance of a Primary Actuator-driven 2.25 mL autoinjector.
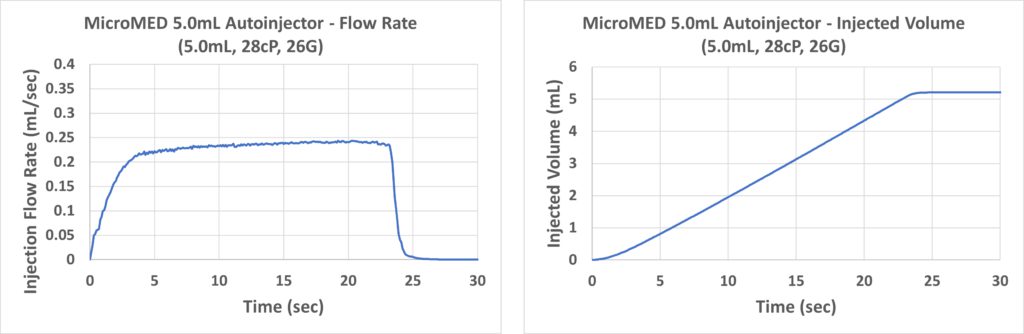
Figure 8: Injection rate (left) and injected volume (right) performance of a Primary Actuator-driven 5 mL autoinjector.
“The Primary Actuator is an innovative, small-footprint, low-cost solution for the delivery of large-volume, high-viscosity biologics.”
Since the rubber plungers used in many primary containers are known for suffering from stiction issues, this real-time injection-rate performance provides a detailed insight into how smoothly the Primary Actuator was able to overcome this challenge. When the resulting data from this test was compared with an off-the-shelf 1 mL autoinjector (Figure 6, right), two obvious differences were observed:
- The Primary Actuator provides a gentle flow increase at the beginning of injection, whereas the commercial autoinjector shows an instant initial spike in flow rate
- The Primary Actuator demonstrated a smoother and more stable flow control after reaching a steady-state flow compared with the off-the-shelf autoinjector, which had an up-and-down variance in flow rate during use.
As previously discussed, spring-driven autoinjectors are known for their high initial impact force when the spring is first released. This problem becomes much more serious in large-volume autoinjectors, such as 2.25 mL, 5 mL or even greater volumes. Therefore, current large-volume autoinjectors with volumes greater than 5 mL cannot use PFSs as their primary container and require pharma companies to use non-standard alternatives, such as prefilled cartridges or custom plastic containers, which regularly results in the US FDA and other regulatory authorities requiring additional tests and reviews, increasing the risk and time-to-market involved in product development. Additionally, line springs – those normally used in autoinjectors – are also known for their varying driving force across the overall injection process and problems related to the plunger sticking, which occur quite often due to misalignment of force in combination with the plunger stiction issue.
Next, MicroMED’s Primary Actuator was demonstrated to be capable of actuating a 2.25 mL PFS with 28 cP simulated fluid through a 26G needle with a total injection time of about 17 seconds. The flow-rate-to-time performance data were collected using a commercial high-accuracy flow sensor (Figure 7, left). It went smoothly from zero to steady-state without an instant initial spike and ceased when the plunger hit the end of the container, showing good end-of-the-injection flow behaviour.
These data were then converted into an injected volume-to-time graph (Figure 7, right). This injected volume curve shows a flat and smooth increase, representing an excellent drug injection performance. These results show that Primary Actuator driven autoinjector products offer a much lower risk of causing pain to the patient, due to the stable flow control, and breaking the glass container, due to the lack of initial impact commonly seen in spring-actuated autoinjectors.
Figure 8 depicts the MEMS engine’s performance driving a 5 mL PFS autoinjector with a 26G needle and containing 28 cP fluid. Similar to the 1 mL and 2.25 mL autoinjectors, there was no initial spike of force and a steady-state stable flow was established. This represents a key signature of the Primary Actuator’s innovative technology. Additionally, the Primary Actuator achieved an injection time of around 23 seconds for the 5 mL injection.
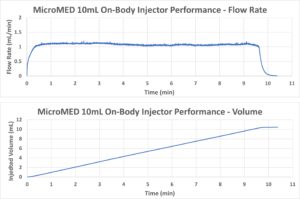
Figure 9: Injection rate (top) and injected volume (bottom) performance of a Primary Actuator-driven 10 mL OBI.
PRIMARY ACTUATOR IN LARGE-VOLUME OBI
A prototype 10 mL OBI with 28 cP drug simulated fluid and a 26G needle cannula was developed using MicroMED’s Primary Actuator to demonstrate a smooth and stable injection control with a target injection time of 10 minutes. Figure 9 shows the results of this test, as measured by a commercial flow sensor. The flow rate (Figure 9, top) was smooth and stable throughout the whole injection process and the converted injection volume (Figure 9, bottom) shows a linear increase, demonstrating extraordinary injection speed control for the MEMS-enabled OBI system.
The total capacity needed from the battery was about 17 mAH and the electrical power needed was 0.4 W, meaning a small commercial lithium ion primary battery would be able to power the micro actuator. This enables designers to simplify the design and lower the overall drug delivery device footprint. Overall, these tests demonstrate that MicroMED’s Primary Actuator is superior to many current injection driving technologies, such as springs and motors (Table 1).
CONCLUSION
The Primary Actuator is an innovative, small-footprint, low-cost solution for the delivery of large-volume, high-viscosity biologics. Unlike springs, the MEMS engine has a gentle push in the beginning, followed by a smooth and stable injection flow control toward the end of the injection. This technology represents a next-generation-device-enabling technology with the potential to solve major challenges in current large-volume SC injector product development.
Enabling device designers to use standard PFSs even at high volumes and viscosities can lead to significant savings in development time and resources for pharma companies. The Primary Actuator has demonstrated its capability as a smart solution for drug delivery that will benefit all stakeholders in the SC injection market. A successful partnership between strategic collaborators and MicroMED will enable this engine technology to be developed into a high quality commercial injector product meeting the real needs of today’s large-volume biologic injectables.
REFERENCES
- Epstein RS, “Payer Perspectives on Intravenous versus Subcutaneous Administration of Drugs”. Clinicoeconomics and Outcomes Res, 2021, Vol 13, pp 801–807.
- “Inspiring New Options”. Halozyme webpage, accessed Aug 2022.
- Badkar AV et al, “Subcutaneous Delivery of High-Dose/Volume Biologics: Current Status and Prospect for Future Advancements”. Drug Des Devel Ther, 2021, Vol 15, pp 159–170.
- “Maggie® 5.0 mL”. SHL webpage, accessed Aug 2022.
- “ArQ® – Bios Subcutaneous Platform”. Oval Medical Technologies webpage, accessed Aug 2022.
- “Stay at Home With Neulasta® Onpro®”. Neulasta Onpro webpage, accessed Aug 2022.
- “How to Take Repatha®”. Repatha webpage, accessed Aug 2022.
- Li B et al, “New Engine for Primary Container Injectors: a Powerful Micro Linear Actuator”. ONdrugDelivery, Issue 117 (Mar 2021), pp 82–85.