To Issue 138
Citation: Bredel T, Zeiss B, “Silicone-Oil-Free, Coating-Free, Tungsten-Free Prefillable Syringes”. ONdrugDelivery, Issue 138 (Oct 2022), pp 70–74.
Taras Bredel and Bernd Zeiss present the studies, development, performance and benefits of the Injecto lubrigone3 plunger stopper combined with the Gerresheimer RTF® glass syringe – a silicone-oil-free, coating-free, tungsten-free, low-particle prefilled syringe solution for sensitive drug products.
“Existing marketed drug products can benefit from an improved product offering by renewing or broadening their delivery format options.”
The prefilled syringe (PFS) market is expected to grow from €6.6 billion (£5.7 billion) in 2021 to €13.1 billion by 2028 at a compound annual growth rate of 10.7%.1 In 2019, there were more than 8,800 injectable drug products in the pharmaceutical pipeline, of which more than 2,500 were protein-based large molecules.2
Developing large-molecule drug products is a complex process, demanding careful planning for the primary packaging and the referred risks over the lifetime of the product. Likewise, existing marketed drug products can benefit from an improved product offering by renewing or broadening their delivery format options.
Increasingly, PFSs are being selected as a delivery format for liquid injectables due to their many significant advantages, including patient safety through the reduced risk of pathogen exposure, dose accuracy, cost benefits (such as product storage and time needed for administration) and the ease of self-administration. Glass is by far the most common material for PFSs due to its many advantages. However, studies indicate that protein loss is significantly increased with siliconised glass syringes compared with silicone-oil-free syringes and that silicone oil increases the surface adsorption capacity of proteins, resulting in insoluble aggregates and the loss of monomers.
Furthermore, studies indicate that the amount of insoluble aggregates can be correlated to the level of silicone oil. Aggregation may occur when proteins bind to the surface of the silicone oil, creating heterogeneous nucleation sites in which the proteins undergo an irreversible change in conformation. When the oligomeric state of the proteins is adversely affected by this cyclic behaviour, the therapeutic effect is degraded and the potential for immunogenic response grows.3-7
According to Martin Gonzalez, PhD, Senior Group Leader, R&D, at Pfizer CentreOne, “Silicone is always a prime suspect in cases of protein aggregation, since it generally interacts with protein and can trigger the aggregation process.”8
Another known source of protein aggregation comes from the tungsten residuals from the pin used to form the needle bore in glass syringes.9,10 To mitigate this, Gerresheimer offers silicone-oil-free glass syringes where the bore has been made with a ceramic pin and hence without tungsten residuals.
Stability and efficacy are major liabilities both during a drug product’s development and throughout its lifetime – and, although different excipients can be combined with the drug product to accommodate stability, the primary packaging, including the container closure, should be a remedy, not a risk-increasing factor.
With the increasing number of large-molecule and ophthalmic drug products, complementary primary packaging is paramount. Gerresheimer’s glass syringes, combined with Injecto’s biocompatible lubrigone3 plunger stoppers, which enable PFSs that are completely free of silicone oil, coating and tungsten, are a key evolution for combining complex drug products with increasingly in-demand ready-to-use delivery formats.
THE CHALLENGE OF REMOVING SILICONE OIL ON PLUNGER STOPPERS AND IN GLASS CONTAINERS
The ISO 11040-4 regulation stipulates a tolerance span of +/-0.1 mm on the 6.35 mm inner dimension of 1.0 mL (long) glass containers, and the conditioned need for an oversized plunger stopper to ensure container closure normally requires the use of a slip agent, such as liquid or baked-on silicone oil, for rubber plungers to be functional.
A study investigating the removal of silicone oil, performed by Gerresheimer, showed that, after only a short period of storage, rubber plunger stoppers would either get stuck (even when applying forces over 40 N) or the sealing elements would be torn and cause a back flow through the plunger stopper sealing elements. After longer storage, the rubber plunger stopper and glass combination allowed for completion of the administration but required forces over 35 N. A glass syringe and rubber plunger stopper without silicone oil would result in dysfunctional behaviour, as seen in Figure 1.
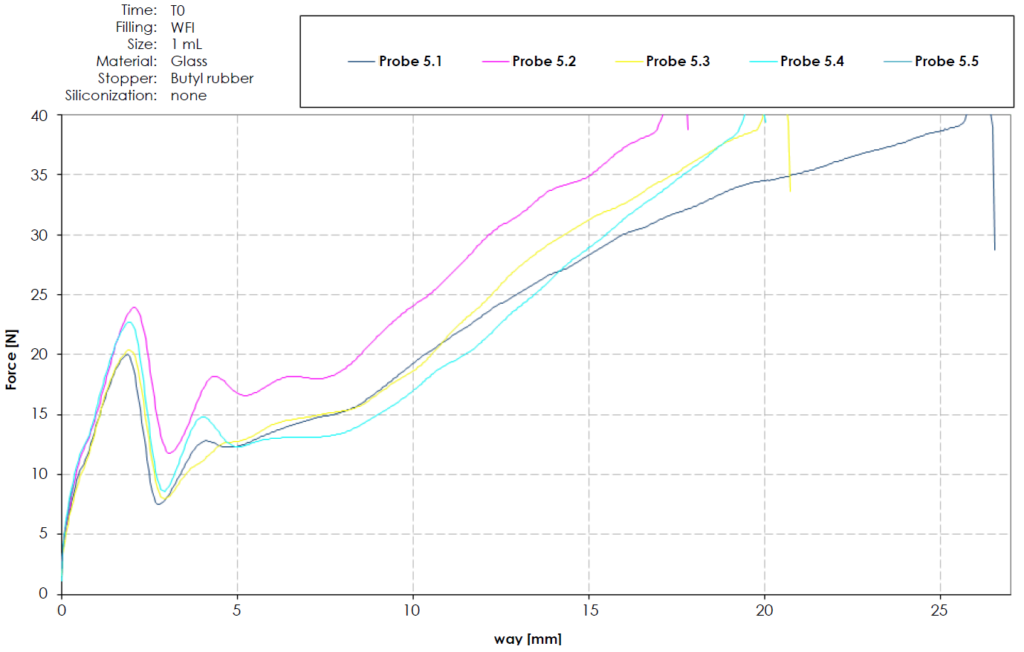
Figure 1: Coated butyl rubber stopper without siliconisation in glass.
A similar challenge with increasing forces can be encountered with siliconised syringes and rubber plunger stoppers over time if the liquid silicone oil migrates to form an uneven coating or cause lubrication depletion.11
Ageing of siliconisation over time may be an issue affecting long-term storage and long-term functionality. This can be particularly important in autoinjectors, which rely on a constant spring force whereas, during manual injection, a healthcare professional would either recognise higher forces and adapt intentionally or reject the syringe.
“During injection moulding, only the exact amount of material needed is injected into the mould cavities in each cycle.”
A DEVELOPMENT BREAKTHROUGH
Using complex modelling and comparison of material performance, compression set, friction coefficient, strain and stress characteristics, Injecto identified the profile and design features needed to resolve the storage and delivery problems without lubrication of the container or plunger stopper (Figure 2).Due to the chosen plunger stopper material’s shore-A hardness, combined with the compression set, it has been possible to design a sealing element with a steep attack angle towards the container wall. This profile reduces the abutting surface, which, combined with the low friction coefficient between the plunger stopper material and glass, accomplishes the balance between container closure integrity and operating forces. The lubrigone3 plunger stoppers are fully functional with both glass and polymer – cyclic olefin copolymer (COC) and cyclic olefin polymer (COP) – syringes, however there is a slightly higher friction coefficient against polymers compared with glass.
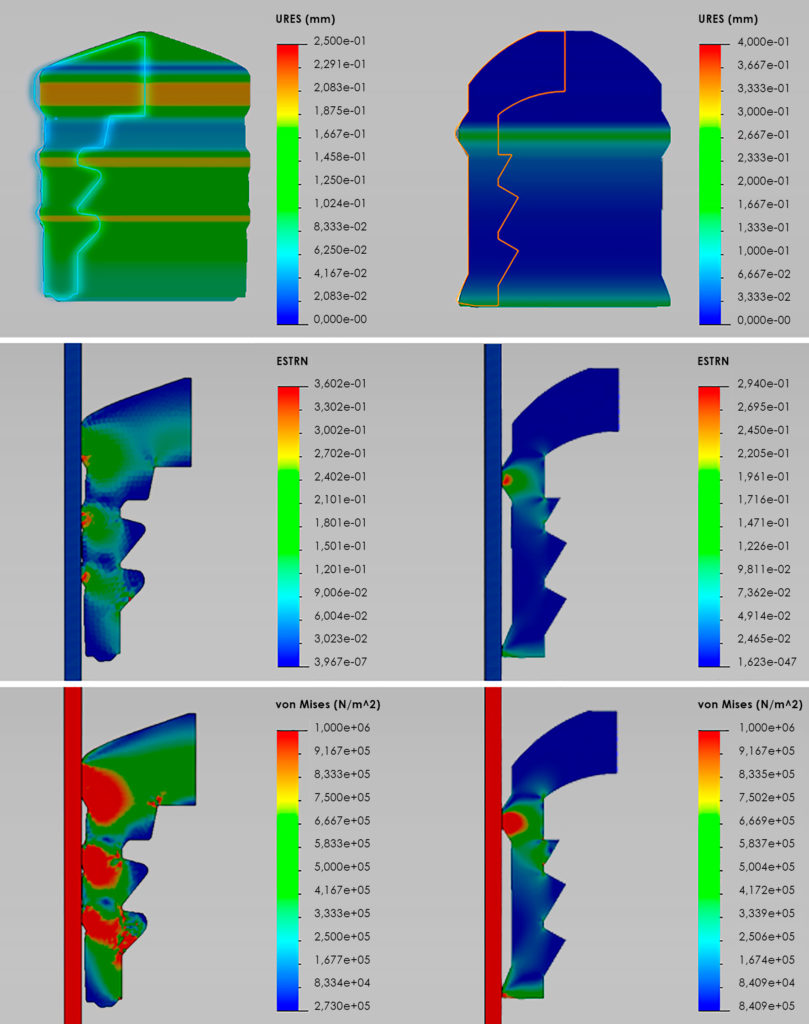
Figure 2: Simulating and comparing strain and stress profiles of unsiliconised lubrigone3 plungers and a coated butyl rubber plunger in glass, in the conversion from dynamic state to motion by combining a nonlinear simulation of hyperelastic using Mooney-Rivlin and a linear elastic deformation using Mohr-Coulomb. Left: Butyl rubber plunger stopper; Right: lubrigone3 plunger stopper; Top: Deformation of plunger stopper; Mid: Maximum strain; Bottom: Maximum stress.
BALANCING CONTAINER CLOSURE INTEGRITY AND FORCES
To accommodate the full span of the container’s inner dimensions, and thereby the larger or smaller outward forces of the plunger’s sealing elements against the container inner wall, the lubrigone3 patented inner mass reduction system was developed to reduce excessive outward forces and thereby provide a more uniform force profile (Figure 3). Furthermore, the precision injection moulding production method ensures low plunger stopper tolerances and thereby reduces the overall tolerance stack-up.
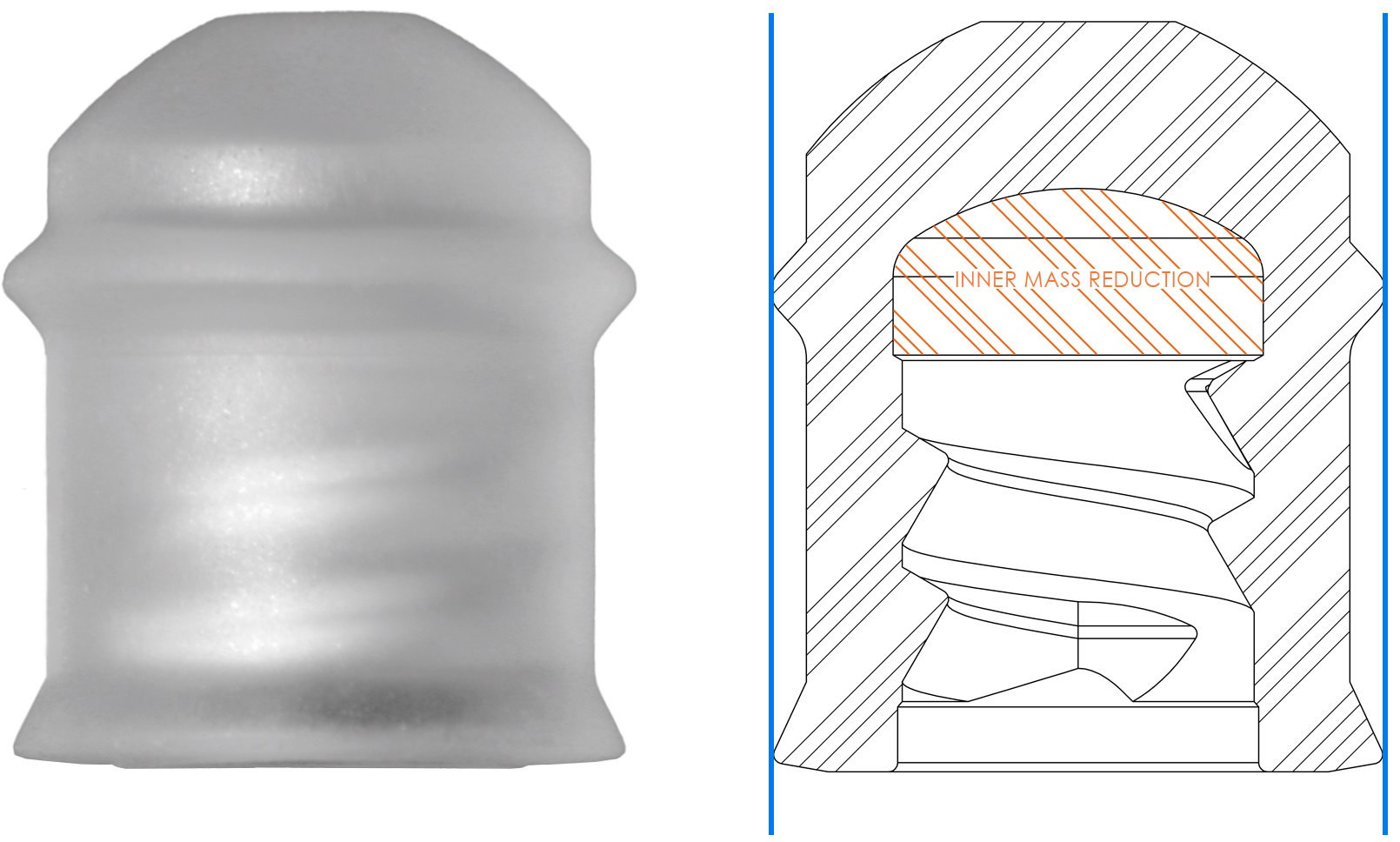
Figure 3: Inner mass reduction.
As such, the material characteristics, the sealing profile and inner mass reduction system enable removal of the silicone oil and/or lubricous coatings, while ensuring that the lubrigone3 plunger stopper accommodates the full tolerance span of container inner diameters stipulated by ISO 11040-4 and providing consistent container closure and operating forces throughout the entire lifetime of the PFSs.
Force Profile for 0.5 mL and 1.0 mL (long)
The lubrigone3 plunger stopper has been force tested with random selected Gerresheimer RTF un-siliconised glass syringes (Figure 4).
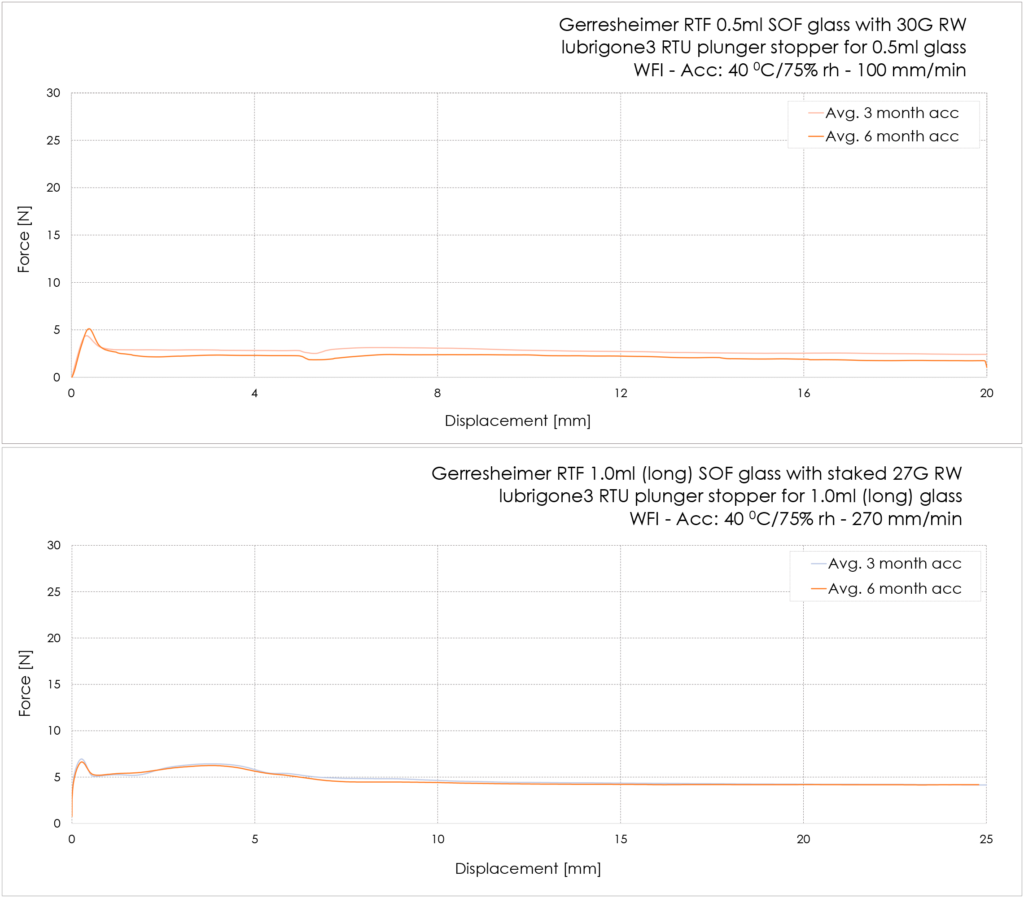
Figure 4: Examples of the break-loose and gliding-force profiles.
The 0.5 mL glass syringes had luer adapter and 30Gx½” needles mounted. The syringes were stored under accelerated conditions for three and six months before testing at 100 mm/min for ophthalmic application. The average break loose was 5.0 N and the average glide force was 2.4 N after three months’ accelerated storage and, respectively, 5.0 N and 2.3 N after six months’ accelerated storage.
The 1.0 mL (long) had a 27G TW staked needle. The syringes were stored under accelerated conditions for three and six months before testing at 270 mm/min. The average break-loose force was 7.3 N and the average extrusion force was 4.6 N after three months’ accelerated storage and, respectively, 7.1 N and 4.6 N after six months’ accelerated storage (Table 1).
| He leak Speed (Pa m3/s) | |
| 1 | 1.2x 10-9 |
| 2 | 1.5x 10-9 |
| 3 | 1.2x 10-9 |
| 4 | 1.3x 10-9 |
| 5 | 1.1x 10-9 |
| 6 | 9.2x 10-9 |
| 7 | 6.5x 10-9 |
| 8 | 1.4x 10-9 |
| 9 | 1.3x 10-9 |
| 10 | 1.0x 10-9 |
Table 1: He leak in large inner-diameter COP containers.
Helium Leak √ PASSED
The lubrigone3 plunger for 1.0 mL (long) has an average 1.03*10-9 Pa m3/s He leak in polymer containers with large inner diameters. With effusive He leak rates of 10-7 Pa m3/s, the probability of microbial ingress approaches zero.
Blue Dye √ PASSED
32 samples were tested, based on Gerresheimer RTF 1.0 mL (long) glass syringes with staked 27G TW cannulas and lubrigone3 plunger stoppers at different time points. 32 additional samples were tested based on Gerresheimer RTF 1.0 mL (long) COP syringes with luer lock adapter and lubrigone3 plunger stoppers at different time points.
BIOCOMPATIBILITY
There are known cases in which the coatings used to create a barrier between the extractables and the butyl rubber plunger stopper have been damaged. This exposes the drug product to direct contact with the butyl rubber, meaning that some of the leachable compounds in the formulation may potentially cause toxicity.
The lubrigone3 thermoplastic elastomer is a special compounded styrene-ethylene butylene-styrene (SEBS) formulation. This is a biocompatible material with no compounds of concern both in water (pH 2.5–9.5) and water with Tween20, and therefore does not require a coating to inhibit adverse extractables. Consequently, there are no risks associated with the challenges and concerns regarding poly- and perfluoroalkyl substance (PFAS)-based coatings. The material has been thoroughly evaluated and tested against the relevant endpoints of ISO 10993-1:2020 Annex A. The material has been validated as gamma irradiation compliant.
PARTICLES
In a subvisible particle study with Gerresheimer RTF syringes, the particle count by light obscuration was 2.9 at >10 μm/mL, 0.03 at >25 μm/mL and 0 at >50 μm/mL, which is substantially below a tenth of the particles allowed by USP<789> for ophthalmics (Figure 5). The particulates have also been evaluated by size exclusion chromatography, light scattering, micro-flow imaging and resonant mass measurement, which confirmed the preservation consistency over time with the omission of silicone oil.
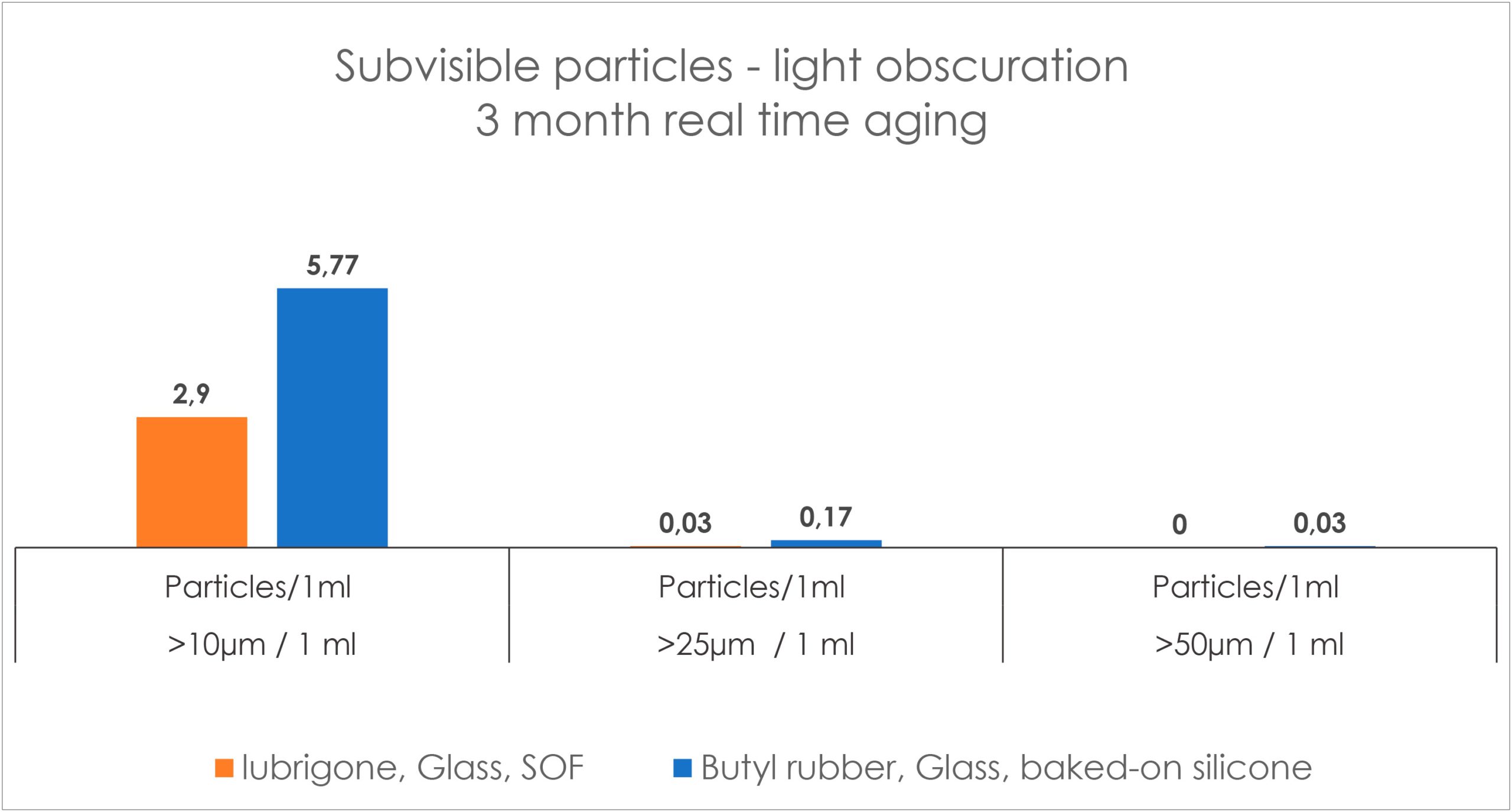
Figure 5: Subvisible particle count in water for injection by light obscuration.
Improved Sustainability
Thermoplastic elastomer plunger stoppers are produced by injection moulding; a highly precise production method that provides many advantages. Firstly, it is a much less resource-intensive production method compared with the compression moulding and vulcanisation associated with processing rubber. The production cycle is typically only a fraction of that for vulcanised rubber. Furthermore, injection moulding is a clean process that outputs sterile parts due to the processing temperature of 170–250°C (338–428°F) and taking place in a Class 7 clean room. There are no hands-on or washing processes involved in the production. The processes for ready-to-use lubrigone3 plunger stoppers are:
- Injection moulding
- Packaging
- Sterilisation.
Secondly, the material waste is minimal. During injection moulding, only the exact amount of material needed is injected into the mould cavities in each cycle. Finally, the lubrigone3 plunger stopper material is 100% recyclable.
COMMERCIAL SUPPLY
The lubrigone3 plunger stoppers are produced under ISO 13485 in Denmark and are supplied in a ready-to-use state by gamma sterilisation (Figure 6). Furthermore, Injecto is involved in several custom-sized plunger stopper developments for both PFSs and medical devices.

Figure 6: Lubrigone3 plunger stoppers.
REFERENCES
- “Prefilled Syringes Market (By Type: Conventional, Safety; By Material: Glass, Plastic; By Design: Single-Chamber, Dual- Chamber, Customized; By Product: Complete Syringe Set, Components and Accessories; By Closing System: Staked Needle System, Luer Cone System, Luer Lock Form System: By End User) – Global Industry Analysis, Size, Share, Growth, Trends, Regional Outlook, and Forecast 2022–2030”. Industry Report, Precedence Research, Mar 2022.
- Lloyd I, “Pharma R&D Annual Review 2019”. Pharma Intelligence, 2019, pp 24–25.
- Chantelau E, Berger M, “Pollution of insulin with silicone oil, a hazard of disposable plastic syringes”. Lancet, 1985, Vol 1(8443), p 1459.
- Krayukhina E et al, “Effects of Syringe Material and Silicone Oil Lubrication on the Stability of Pharmaceutical Proteins”. J Pharm Sci, 2015, Vol 104(2), pp 527–535.
- Chi E et al, “Heterogeneous nucleation-controlled particulate formation of recombinant human platelet-activating factor acetylhydrolase in pharmaceutical formulation”. J Pharm Sci, 2005, Vol 94(2), pp 256–274.
- Thirumangalathu R et al, “Silicone Oil- and Agitation-Induced Aggregation of a Monoclonal Antibody in Aqueous Solution”. J Pharm Sci, 2009, Vol 98 (9), pp 3167–3181.
- Gonzalez M, ”Is Your Biologic At Risk For Protein Aggregation? Part 3”. Pfizer CenterOne Contract Manufacturing, Jul 2018, pp 1–3.
- Liu W et al, “Root Cause Analysis of Tungsten-Induced Protein Aggregation in Pre-filled Syringes”. PDA J Pharm Sci Technol, 2010, Vol 64(1), pp 11-19.
- Jiang Y et al, “Tungsten-induced protein aggregation: solution behavior”. J Pharm Sci, 2009, Vol 98(12), pp 4695–4710.
- Maggio E, “Use of excipients to control aggregation in peptide and protein formulations”. Journal of Excipients and Food Chemicals, 2010, Vol 1(2) pp 40–49.
- Gregory S et al, “Practical fundamentals of glass, rubber, plastic sterile packaging systems”. Pharm Dev Technol, 2010, Vol 15(1), pp 6–34.

