To Issue 141
Citation: “Connected Drug Delivery – Is the Near-Term Opportunity in Clinical Development? – an Interview with Oliver Eden, Jabil Healthcare”. ONdrugDelivery, Issue 141 (Dec 2022), pp 27–30.
Oliver Eden of Jabil Healthcare looks at the factors influencing the return on investment metrics in clinical development and introduces Jabil’s autoinjector platform Qfinity™ and its connected version, Qfinity+™.
“Improving patient engagement is critical for reducing study delays
and accelerating product development cycle time.”
Q To begin, can you give us an overview of the challenges facing pharma with respect to return on investment?
A An urgent challenge across the healthcare ecosystem is how to accelerate the development of safe, efficacious medicines that improve health outcomes for patients. For every one asset in Phase I trials that becomes an approved medicine, there are nine that will not. Costs for developing a new molecular entity can run into the billions. Although return on investment metrics depends upon a variety of factors, improving patient engagement is a primary concern for pharma within clinical development. Connected health helps to mitigate challenges with patient retention and adherence to study protocols, thereby supporting accelerated timelines, but in order for these benefits to be fully realised, connectivity must be executed with a patient-centric mindset and, critically, at a price point that is not a barrier to adoption.
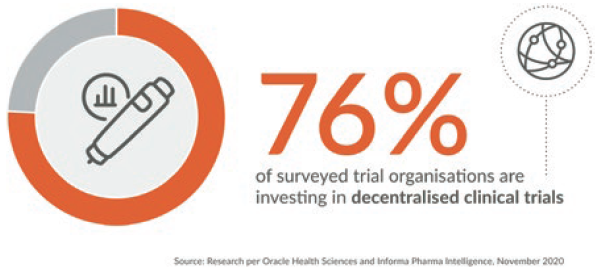
Figure 1: There has been a shift in clinical trials towards a more virtual and decentralised structure.
Q What are the macro trends or other considerations driving development of autoinjector platform solutions?
A Potentially significant tailwinds are in play for pharma customers aligning their drug development strategies with the following two emerging industry macro trends. Firstly, there is an increasing orientation within pharma pipelines towards biologics, which must be administered via a drug delivery device, such as an autoinjector. And secondly, there is a shift in clinical trials, particularly in the post-covid-19 landscape, towards a more virtual and decentralised structure, which is driving greater reliance upon connected technology solutions (Figure 1). Together, these trends are driving growth in self-administered subcutaneous drug delivery via an autoinjector and an increase in the use of connected solutions.
Q Do you see the role of digital health solutions increasing in clinical development?
A Yes, absolutely. As a contract manufacturer to major healthcare brands across the industry, Jabil’s engineers and technicians work on the industry’s transformation to digital healthcare every day. In fact, about 50% of the healthcare products Jabil manufactures have a digital component, and this is rising by around 10% each year. So, to offer a product that meets current and future market demand, especially for the dynamic decentralised clinical trials (DCTs) market, we must consider how it will collect, connect and deliver.
Q What is the outlook for digital solutions in clinical development and commercial supply?
A The near-term opportunity for digital health solutions in clinical development is particularly compelling. Pharma research and development programmes continue to face sub-optimal recruitment and retention performance, along with other study management challenges, opening a line of sight to the value proposition for what digital can provide.
Viewed through a monetisation lens, connectivity’s value in clinical trials is demonstrable and quantifiable, delivering improved patient engagement and retention. This leads to shorter and/or smaller studies, while also supporting more informed decision making regarding programme advancement or termination.
The valuation model for connectivity in commercial supply is also attractive but hinges on less transparently quantifiable adherence, compliance and persistence performance metrics, as well as challenges in defining digital health’s impact on healthcare outcomes and total healthcare costs. If deploying connected solutions in clinical development can be demonstrated to improve patient engagement – delivering the value proposition described – it will become easier to quantify the impacts upon patient behaviour that would drive value in commercial supply.
Q How does a connected autoinjector deliver value in clinical development?
A Based on participation surveys and studies reviewed from the Tufts Center for the Study of Drug Development (MA, US) and research from Global Data (London, UK), approximately 80% of studies are delayed by as much as six months, and 20% of clinical trials fail due to insufficient patient enrolment. These shortfalls are costly. Improving patient engagement is therefore critical for reducing study delays and accelerating product-development cycle time.
Incorporating a connected autoinjector in clinical studies provides investigators with a valuable tool for capturing patient insights, enabling them to manage study participants more effectively to adhere to the protocol. When study investigators are freed up to focus their efforts primarily upon those study participants who – for a variety of reasons – are struggling to follow the study protocol, it helps improve retention metrics and other key performance indicators.
Q Can you quantify the potential opportunity provided by connectivity?
A With reference again to the Tufts studies and other research that projects costs for developing a new molecule at circa US$2.6 billion (£2.3 billion) with only a 12% probability of success. Improving the odds by just 1% through the improved decision made possible by a connected autoinjector’s adherence/compliance data would equate to saving around $120 million.
Connected devices facilitate real-time data collection, providing sponsors with increased actionable information earlier in a trial regarding which assets should be progressed or terminated. There is significant value in optimising how these decisions are made.
Additionally, consider how connectivity’s improvement of patient engagement supports both smaller studies and shorter study timelines. Patient recruitment and retention accounts for nearly 24% of a study’s total cost, so for an asset with peak sales of $1 billion per annum, shortening the development process projects to an increase in total sales of approximately $85 million per month.
“Connected devices facilitate real-time data collection, providing sponsors with increased actionable information earlier in a trial regarding which assets should be progressed or terminated.”
Q What does Jabil Healthcare’s connected platform autoinjector measure?
A Qfinity+ captures adherence, compliance and persistence data. In other words, connectivity provides critical insight into how well or easily a participant can execute the clinical study per protocol. Additionally, Qfinity+ measures injection duration, confirming whether a full dose is delivered.
Integrating these data with additional connected sources, such as data from wearables, can provide a broader set of values for assessing safety signals around a dosing event, such as heart rate and longitudinal measurement of activity or sleep, which may be associated with efficacy.
Q Why did you develop both connected and non-connected versions of the autoinjector platform?
A The intention with the Qfinity platform solutions is to offer the market an autoinjector, with an option for a connected version, in the same form factor and with the same user operation steps (Figure 2). The connectivity feature benefit in Qfinity+ is seamless, recording dosing events and transmitting the data without any additional user steps (or training requirement) versus the non-connected version, Qfinity.
Ease-of-use for patients and caregivers has been Jabil’s goal throughout the development of these platforms. Providing a more sustainable solution has also been intentional, which is why Jabil chose to develop Qfinity and Qfinity+ as reusable autoinjector platforms. Incorporating electronics into the reusable injector unit delivers connectivity for Qfinity+ that does not impose additional patient burden, and at a cost that is comparable with an unconnected, disposable autoinjector.

Figure 2: The Qfinity platform offers the market an autoinjector with an option for a connected version in the same form factor and with the same user operation steps.
Q Why do the connected and non-connected autoinjectors look and feel identical?
A From the outset of the development process, Jabil focused on finding the best way to ensure that Qfinity+’s additional feature benefit – connectivity – does not impose any additional burden on patients or caregivers. The company intentionally designed Qfinity+ with the electronics integrated within the same form factor as the non-connected version. For the user, this minimises patient burden because they follow the same steps and grip function without requiring additional training.
Q Was consideration given to potential user transitions between connected and non-connected versions?
A Absolutely. Even though today’s shift towards more virtual, decentralised or hybrid clinical studies drives greater reliance upon connected technology solutions, it is important to realise that connectivity may not always be required when that product comes to market.
The design challenge then becomes how to ensure minimal impact in terms of user experience for participants transitioning between versions of the autoinjector without the need for retraining. This solution, informed throughout by patient-centricity, simplifies the user experience and allows for much greater ease moving between versions.
For example, it would be possible to conduct a DCT with Qfinity+ and then launch the product commercially with Qfinity because the user steps are the same. Similarly, the two platforms’ shared design enables the transition of a trial participant from Qfinity to Qfinity+ allowing healthcare providers the opportunity, as needed, to engage more effectively with those patients who may be struggling to adhere/comply with their treatment plan.
Q What is the connection protocol – how does the connected autoinjector transmit data?
A Many market-leading autoinjector platforms require smartphones for data transmission. While seemingly convenient, smartphone penetration varies significantly by geography, demographics and socio-economic status (Figure 3). Even in an advanced country like the US, smartphone penetration in those over 65 years old – a key healthcare market – is only 61%. Throughout the developed and developing world, coverage gaps exist and must be considered.
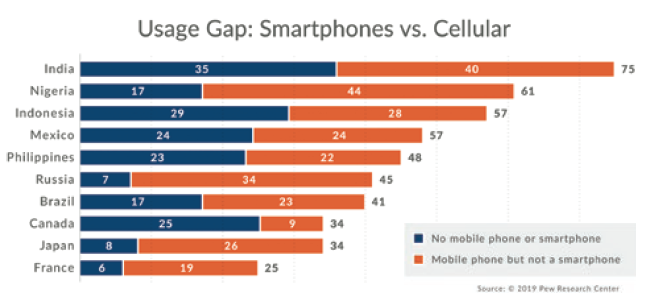
Figure 3: Smartphone penetration varies by geography, demographics and socio-economic status.
Therefore, integrated cellular communication was chosen for the Qfinity+ platform. It is the most inclusive connectivity solution providing more than 95% coverage worldwide. This feature also supports the broader strategic objective of pharma companies and regulators to recruit more representative patient populations for trials.
Q How is the Qfinity+ platform more inclusive and accessible?
A The Qfinity+ platform does not require a smartphone or wi-fi and connectivity is enabled without touchscreens, apps or other interfaces. The only difference for the user is the addition of a home-hub base. When the user places the Qfinity+ drive unit into the home hub, that action seamlessly transfers the data to the cloud while simultaneously charging and safely storing the device for the next use, meaning that both these critical requirements of a digital healthcare device’s performance are accomplished with a single step (Figure 4).
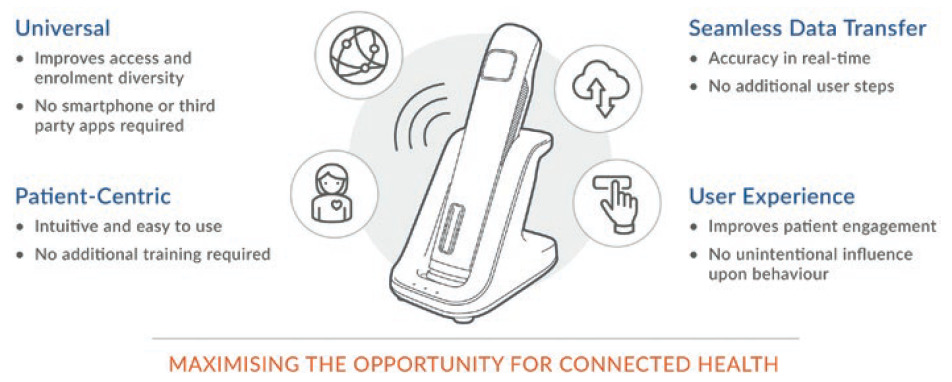
Figure 4: The benefits of a cellular home hub.
“The evidence is already compelling and will continue to build: connected technology is a key component for enabling decentralised trials.”
Q Do participants require any additional training for the connectivity feature?
A No. The dosing event ends when the patient places the autoinjector into the home hub. This is a very simple and intuitive solution for patients and caregivers, in contrast with some market leading single-use autoinjectors that use a reusable electronic sleeve. The downside of an add-on sleeve solution, particularly in clinical studies, is the potential it has for changing the user experience with training, user steps and grip function as well as, possibly more importantly, the way it signals to participants that they are being monitored.
Likewise, some solutions require study participants to interact with an external app to push data to a smartphone. These userengaging steps serve as overt triggers and can potentially influence behaviour.
Q How do you see patients and caregivers responding to your product choices?
A The attributes Jabil set out to include have been validated through a series of formative human factors studies. The Qfinity platform compared favourably in a compromised patient population (rheumatoid arthritis), with reference to two market-leading disposable single-use autoinjectors.
Q Any additional comments on the opportunities in today’s market for a connected autoinjector?
A Recently, the Tufts Center for the Study of Drug Development and Medable (CA, US) have been collaborating on a comprehensive return on investment analysis for conducting DCTs. The initial narrative calls attention to the dramatic reduction in cycle times and cost savings for sponsors using DCT technology as part of their protocols. The evidence is already compelling and will continue to build – connected technology is a key component for enabling decentralised trials.
Jabil understands that there are choices and options for pharma customers seeking to meet challenging objectives. Solutions, ideally, need to work for all stakeholders, and they also need to work in the real world. This is where the company started its work – the prospect of aligning form with function for a more seamless and inclusive patient journey, greater value for pharma customers and better health outcomes for the patients they serve.

