To Issue 167
Citation: Cusack A, Nazarzadeh E, Pritchard J, “Innovative Acoustic Nebuliser Technology”. ONdrugDelivery, Issue 167 (Nov 2024), pp 28–32.
Andrea Cusack, Elijah Nazarzadeh and John Pritchard share insights into Nebu-Flow’s innovative nebuliser platform, designed to overcome the technical restrictions of current technologies that limit the delivery of advanced medicines to the lungs. They also share an update on the development of new digital features for the Nebu-Flow product range.
Respiratory diseases, such as asthma and chronic obstructive pulmonary disease (COPD), are major causes of death and disability worldwide, despite medical advancements.1 Researchers face two types of challenges. Firstly, there is a treatment gap for respiratory disorders, with most medicines focusing on symptom relief and preventing deterioration rather than providing an effective treatment.2,3 Secondly, selection of the delivery route and correct drug delivery device has proven to be a challenge in the treatment of respiratory disorders.
Although the best route of delivery for respiratory treatment is the direct delivery of drugs to the lungs through the inhalation route, the lack of a high-performing device, coupled with the low efficacy of drugs, usually results in selection of the parenteral route. This route usually requires a higher dose of medicine, which can result in higher exposure and toxicity. To address these challenges, clinicians and researchers are seeking optimal drug formulations and delivery devices to target specific lung areas. However, selection of an inhalation drug delivery device relies on several factors:
The API, Formulation and Dosage
Inhalation formulations are usually in powder or liquid form. Powder formulations are delivered through dry powder inhalers (DPIs) and suitable for molecules that can be micronised or formulated in powder form and lower doses. Liquid formulations can be delivered by metered dose inhalers (MDIs), soft mist inhalers (SMIs) or nebulisers. Both MDIs and SMIs are suitable for delivery of lower volumes of formulations, whereas nebulisers are the best device for flexible volumes. Delivery of biological formulations is of significant interest. Most are synthesised as an aqueous or ethanol solution/suspension and so are easily formulated for nebulisation. However, there are extremely limited solutions available for inhalation delivery of these fragile molecules.
“While inhalers are portable devices that suit an active lifestyle, their correct use requires a high degree of co-ordination between actuation and inhalation.”
Ease of Use and Target Patient Group
While inhalers are portable devices that suit an active lifestyle, their correct use requires a high degree of co-ordination between actuation and inhalation. On the other hand, nebulisers can be used with tidal breathing and are easy to use, which makes them the best device for old and young patients and those who suffer from acute respiratory disorders.
Sustainability
The carbon footprint associated with respiratory disorders is extremely high. As an example, the annual carbon footprint of asthma and COPD exacerbation in the UK is equivalent to one million tonnes of CO2.4 In particular, the propellant of MDIs is a powerful greenhouse gas (accounting for 3% of the UK NHS carbon footprint) and due to be phased out by 2050 in the EU. Only a limited range of formulations can be delivered with new propellants, which results in a gap when it comes to the correct device for inhalation delivery of other formulations.
Digital Connectivity
Digital connectivity is becoming an integral part of the healthcare system, with the aim of improving adherence and clinical outcomes. While inhalers are mechanical devices, nebulisers are electrical devices that can be easily enhanced with digital features.
Inhalation drug delivery depends on aerosol droplet size; droplets larger than 10 μm are trapped in the nose and throat, while those under 5 μm can reach the small bronchi and alveoli, where drug absorption is more effective.5
“Current devices struggle to produce aerosols smaller than the 2 μm needed for effective alveolar targeting.”
Additionally, the large surface area of the lungs offers the potential for systemic drug delivery, presenting inhalation as a viable alternative to oral and parenteral routes. However, current devices struggle to produce aerosols smaller than the 2 μm needed for effective alveolar targeting (Figure 1).6,7
Figure 1: The main three challenges of delivering biologics to the lung – aerosolisation, transport across the mucosa, and uptake and release in the cell.
NEBULISERS AND UNMET NEEDS
This article explores how acoustic wave technology can provide a disruptive solution, addressing the target product profile for a larger number of medicines in inhalation drug delivery, focusing on innovative digital healthcare solutions and next-generation nebulisers for biologics.
As highlighted above, nebulisers are effective in aerosolising and delivering medication during tidal breathing, making drug delivery simpler and eliminating the need for specialised inhalation training. This method also allows for easier formulation adjustments, facilitating dose-ranging studies, which are crucial in the early stages of drug development, thereby accelerating clinical trials and regulatory approvals. This makes nebulisers the ideal drug delivery device for the early stages of drug development, in particular biologics, which are synthesised as an aqueous or ethanol solution/suspension, enabling timeline acceleration to clinical development and market launch.8
In recent years, the use of nebulisers in clinical trials has grown significantly. PharmaCircle reported that 19% of inhaled products in clinical trials in 2019 used nebulisers, which increased to 39% in 2021 and, in August 2023, 42% of clinical trials were using nebulisers.
However, despite the ongoing developments of nebuliser technologies, there are still significant unmet needs. Not only may complex formulations lose potency, the output rate and aerosol droplet size of nebulisers depend on the physical properties of the formulation, such as viscosity and surface tension.9 Furthermore, suspension formulations are unsuited to ultrasonic nebulisers and may block the micron-sized pores in mesh nebulisers.9 In addition, the droplet size is a fundamental function of the design of a nebuliser and can be difficult to change, which can clearly impact the efficacy of the product by targeting the wrong location in the lungs (e.g. nebulised surfactant).10
“Nebu-Flow’s patented technology uses ultrasound waves to disrupt the interface of a liquid volume into an aerosolised mist.”
Nebu-Flow’s patented technology uses ultrasound waves to disrupt the interface of a liquid volume into an aerosolised mist. By coupling acoustic waves with an array of microstructured wells containing liquid, one can control the aerosol droplet size <5 μm, thereby increasing the inhalable fraction of the aerosol for improved deposition in the lung.
Nebu-Flow microstructures are based on confinements of hundreds of micrometres in size, which do not block or denature fragile biologics. Additionally, the technology has the potential to generate aerosols with different median mass aerodynamic diameters (MMADs) from the same device, providing a new solution to pharmaceutical companies for targeted delivery of drugs from the same platform.
DIGITAL HEALTH
Inconsistent medication adherence among patients with chronic illnesses presents a major challenge in healthcare. Poor compliance not only leads to negative health outcomes but also increases costs. While smart medical devices may not directly impact patient readiness, they offer features that can monitor and verify medication intake, although it should be noted that smart devices do not necessarily identify which medicine has been taken. However, they do enable healthcare providers to make better-informed decisions about medication management and therapy adjustments.
Today’s medical device market demands the most up-to-date technology, with connectivity to the cloud now considered a “must have” capability for digital healthcare product solutions. Designing for success means that careful consideration must be given to both the mechanical and physical components as well as the electronic components, thereby creating an electromechanical interface (Figure 2). Nebulisers are using electronic drive systems that can easily be integrated with digital capabilities, compared with pure mechanical devices such as DPIs.
Figure 2: Schematic illustrating the electro-mechanical interface.
Nebu-Flow has developed a method for identifying formulations loaded into a nebuliser, which can be integrated into digital healthcare systems for improved disorder management. This harnesses the unique compositions of commercial inhalation drug formulations, which influence their acoustic properties, such as compressibility. By measuring sound waves travelling through the drug reservoir, these properties can be indirectly assessed. Using an ultrasonic technique, Nebu-Flow has successfully identified various antibiotic and common respiratory drug formulations by analysing attenuation and phase velocities. Combining this technique with additional nebuliser features, such as biometric authentication and tracking of breathing patterns and usage times, can provide reliable data on dose administration, medication types and prescription management.
TUNEABLE NEBULISER OUTPUT
A tuneable nebuliser would significantly enhance the efficacy and safety profiles of new drugs. It has been shown that, by coupling acoustic waves with an array of microstructure, the aerosol droplet size can be controlled within the inhalable range (i.e. <5 μm).11,12 More recently, it has been reported that the aerosol droplet can be controlled by changing the driving parameters, while using the same hardware.13
With a greater understanding of the electromechanical interfaces, Nebu-Flow has tested the technology to ensure that the unmet needs of future nebulised drug products can be met, delivering aerosols with MMADs well below 5 μm,14 enhancing aerosol drug delivery and increasing the fine particle fraction (Table 1).
| Solution | MMAD (μm) | FPF(%) (<5 μm) |
| 50% Ethanol | 1.6 | 82% |
| 1% Sodium chloride | 1.3 | 97% |
| Ventolin 5 mg/mL | 1.4 | 95% |
Table 1: Low MMAD and a high percentage of fine particle fraction (FPF).
“Generation of aerosol during the inhalation period can provide several advantages, such as a reduction in drug delivery time, dosage and passive exposure to a drug.”
SENSING THE BREATH
Generation of aerosol during the inhalation period can provide several advantages, such as a reduction in drug delivery time, dosage and passive exposure to a drug. This is usually implemented through a sensor to detect the breathing pattern, which is embedded within the device. However, the failure of the sensor would make the device redundant.
A modular approach to nebuliser design offers a sustainable solution by enabling the replacement and upgrading of individual components, thereby extending the product’s life. Breath-actuated nebulisers (BANs) represent a significant leap forward in respiratory therapy, enhancing drug delivery efficiency and effectiveness.15,16 Key advancements, such as precise dosing, reduced drug wastage, minimised aerosol generation and improved user experience, are driving the development of next-generation smart nebulisers. However, existing breath-sensing systems are typically integrated into the main device, meaning that any sensor failure necessitates full replacement or costly repairs.
The Nebu-Flow team has explored a modular BAN design that incorporates a simple, cost-effective and robust sensor with sustainability in mind. Paired with Nebu-Flow’s nebuliser technology, this sensor successfully differentiates between inhalation and exhalation cycles. Its sensitivity enables nebulisation to be triggered for tidal volumes ranging from 155 to 900 mL, with a minimum response time of 20 ms for larger volumes.
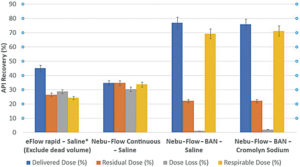
Figure 3: The delivered, residual and respirable doses, as well as dose loss, are presented as percentages of the total dose loaded into the nebulisers. Results are expressed as the mean ± standard deviation. Non-BAN and BAN are compared.
Nebu-Flow is developing a new concept for a modular mouthpiece, using a piezoelectric breath sensor that can increase the delivered and respirable dose drastically compared with non-BANs,while offering an effective, sustainable and customisable solution for enhancing inhalation drug delivery devices (Figure 3). Nebu-Flow has successfully demonstrated that BANs can be both simple and cost-effective to integrate, while simultaneously improving drug delivery efficiency.
BIOLOGICS
Beyond this, one of the major advantages of Nebu-Flow technology is its ability to aerosolise biological entities without denaturing them. This has been tested with a range of biologics, including herring sperm DNA and small interfering ribonucleic acid (siRNA).12 Gene therapy has shown promise to end palliative treatments by addressing the underlying causes of lung dysfunction and infection.17 Gene silencing through siRNA has the potential to deliver on that promise, as evidenced by the number of clinical studies (>24 in 2023) and the increase in US FDA-approved siRNA therapeutics (~5 in 2023).18
Given the challenges associated with targeting the lung for drug delivery,19,20 there is currently an absence of siRNA drugs, with only two trials having progressed to the clinical stage. However, there have been developments in siRNA modification and conjugation to improve stability as well as pharmacokinetic behaviour in vector-free approaches (increased bioavailability in the lung).21
In this study, Nebu-Flow generated ultrafine aerosols laden with vector-free Silencer glyceraldehyde-3-phosphate dehydrogenase (GAPDH) siRNA using Nebu-Flow’s patented technology, which enables aerosol particle size tuning for deep lung drug deposition (Figure 4).10 Nebu-Flow characterised the aerosols and investigated the degradation and biological activity of nebulised siRNA in vitro.
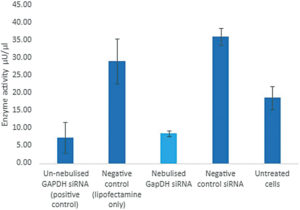
Figure 4: Successful nebulisation and delivery of GAPDH siRNA – the siRNA is active post nebulisation (cyan bar) with no adverse effects to cells observed.
CONCLUSION
Inhaled drug delivery is the primary route for treatment of respiratory disorders, which also shows exciting potential to unlock needle-free systemic drug delivery while reducing drug side effects. Nebu-Flow’s patented nebuliser facilitates the production of ultrafine aerosol particles carrying siRNA. However, ultrasound transducer-based nebulisers may impact drug stability through cavitation, hydrodynamic shear or temperature changes. Duplex siRNA may denature and undergo non-enzymatic hydrolysis, particularly at elevated temperatures.
The Nebu-Flow study illustrates that nebulised siRNA retains its full biological activity, enabling effective delivery to the peripheral and alveolar regions of the lung. The further ability of the technology to control the aerosol droplet size for deep lung deposition with MMAD in the range of 1 μm can provide a significant improvement for efficient inhalation drug delivery, as well as unlocking systemic drug delivery by deposition of drugs in the alveolar region. A combination of technical capabilities and patent protection makes Nebu-Flow an outstanding platform for the development of tailored drug-nebuliser products.
REFERENCES
- James SL et al, “Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017”. Lancet, 2018, Vol 392, pp 1789–858.
- Venkatesan P, “GOLD COPD report: 2023 update”. Lancet Respir Med, 2023, Vol 11(1), p 18.
- Ture DA et al, “Health Literacy and Health Outcomes in Chronic Obstructive Pulmonary Disease Patients: An Explorative Study”. Front Public Health, 2022, Vol 10, article 846768.
- Kponee-Shovein K et al, “Carbon footprint and associated costs of asthma exacerbation care among UK adults”. J Med Econ, 2022, Vol 25(1), pp 524–531.
- Darquenne C, “Deposition mechanism”. J Aerosol Med Pulm Drug Deliv, 2020, Vol 33(4), pp 181–185.
- Martin AR, “Regional deposition: Targeting”, J Aerosol Med Pulm Drug Deliv, 2021, Vol 34(1), pp 1–10.
- Laube BL, “The expanding role of aerosols in systemic drug delivery, gene therapy and vaccination: an update”. Transl Respir Med, 2014, Vol 2, p 3.
- “Biologics Fill Finish Manufacturing Market”. Roots Analysis, 2024.
- Elphick M et al, “Factors to consider when selecting a nebulizer for a new inhaled drug product development program”. Expert Opin Drug Deliv, 2015, Vol 12(8), pp 1375–1387.
- Clark AR, “Essentials for aerosol delivery to term and pre-term infants”. Ann Transl Med, 2021, Vol 9 (7), pp 594–603.
- Nazarzadeh E et al, “Confinement of surface waves at the air-water interface to control aerosol size and dispersity”. Phys Fluids, 2017, Vol 29, pp 112105–112110.
- King X et al, “Ultrasonic Surface Acoustic Wave platform for targeted pulmonary delivery of nano drug vehicles”. IEEE IUS 2019, pp 699–701.
- Macdonald EK et al, “Tuneable Nebulizer Platform for Targeted Inhalation Drug Delivery”. Respir Drug Deliv, 2023, pp 291–294.
- Fullarton N et al, “Ultrafine siRNA-Laden Aerosol using Next Generation Nebulizer”. RDD Online 2024.
- Barjaktarevic IZ, Milstone AP, “Nebulized Therapies in COPD: Past, Present, and the Future”. Int J Chron Obstruct Pulmon Dis, 2020, Vol 15, pp 1665–1677.
- “AeroEclipse BAN Nebulizers Study Summary”. Trudell Medical International, 2023.
- Hu B et al, “Therapeutic siRNA: state of the art”. Signal Transduct Targeted Ther, 2020, Vol 5(1), p 101.
- Gao J et al, “Progress in nonviral localized delivery of siRNA therapeutics for pulmonary diseases”. Acta Pharm Sin B, 2023, Vol 13(4), pp 1400–1428.
- Newman SP, “Drug delivery to the lungs: challenges and opportunities”. Ther Deliv, 2017, Vol 8, pp 647–661.
- Zhang H et al, “Nucleic acid degradation as barrier to gene delivery: a guide to understand and overcome nuclease activity”. Chem Soc Rev, 2024, Vol 53, pp 317–360.
- Hariharan VN et al, “Divalent siRNAs are bioavailable in the lung and efficiently block SARSCoV- 2 infection”. PNAS, 2023, Vol 120(11), article e2219523120.

