To Issue 168
Citation: Schneider A, Lange J, “The Rise of Large-Volume Subcutaneous Administration of Biopharmaceuticals, and Delivery Implications”. ONdrugDelivery, Issue 168 (Jan 2025), pp 10–15.
Andreas Schneider and Jakob Lange consider the subcutaneous administration of larger volumes of biopharmaceuticals in the pharmaceutical industry and summarise a recent study that reviews clinical-stage and approved intravenous and subcutaneous biopharmaceuticals to provide valuable guidance for new product development, as well as for marketing and commercialisation strategies in the rapidly evolving large-volume subcutaneous landscape.
The evolution of biopharmaceuticals has profoundly impacted healthcare, offering innovative treatments that address previously unmet medical needs. Advances in monoclonal antibodies, bispecific antibodies, antibody-drug conjugates, oligonucleotides and other protein-based therapeutics have led to greater specificity and efficacy in targeting complex diseases. These innovations, coupled with the high approval rates and commercial success of antibody-based drugs, have underscored the potential of biopharmaceuticals to transform patient outcomes across oncology, autoimmune disorders and other chronic conditions.
Traditional delivery methods, however, often present barriers to optimal patient care. Intravenous (IV) infusions – a dominant delivery route for biopharmaceuticals – are typically administered in acute-care settings due to safety concerns and dosing requirements that exceed the capacity for subcutaneous (SC) injection. These IV regimens often demand extended administration times – sometimes exceeding several hours – and impose significant logistical and resource burdens on healthcare systems and patients alike.
“By enabling care to shift from hospital settings to outpatient clinics, or even home environments, SC administration offers notable advantages, including shorter injection times and reduced healthcare costs.”
To alleviate these challenges, SC drug delivery has emerged as a practical, patient-friendly alternative. By enabling care to shift from hospital settings to outpatient clinics, or even home environments, SC administration offers notable advantages, including shorter injection times and reduced healthcare costs. A prominent example of this transition is DARZALEX FASPRO® (daratumumab, Janssen), which replaced its IV formulation requiring hours-long infusions with a SC injection that can be completed in under five minutes. This transition not only improved the efficiency of care delivery but also demonstrated the broader viability of SC delivery for biopharmaceuticals.
Industry efforts to expand SC delivery have prioritised the development of large-volume dosing options that accommodate growing therapeutic payloads without compromising the patient experience. While handheld autoinjectors currently support volumes of up to 2 mL with administration times of less than 15 seconds, ongoing research aims to push these limits further. Innovations such as permeation enhancers (e.g. hyaluronidase) and high-concentration formulations are paving the way for SC injections that exceed traditional volume thresholds. In tandem, devices such as on-body injectors (OBIs) and tethered syringe pump drivers are gaining traction for larger doses ranging from 2 to >30 mL, enabling patients to manage treatments independently.
Despite these advances, critical gaps remain in understanding the optimal volume and frequency parameters for large-volume SC (LVSC) biopharmaceuticals. Addressing these gaps is essential for developing next-generation delivery systems and ensuring the seamless integration of LVSC therapeutics into clinical practice. This article explores the trends, challenges and opportunities shaping the future of LVSC biopharmaceuticals, drawing on insights from the study, “Navigating Large-Volume Subcutaneous Injections of Biopharmaceuticals: A Systematic Review of Clinical Pipelines and Approved Products”.1
STUDY OVERVIEW: TRENDS AND INSIGHTS
The study employed a four-step review process to identify approved and clinical-stage biopharmaceuticals requiring LVSC (>2.0 mL) maintenance doses and their expected dosing intervals. The research analysed 1,338 approved and clinical-stage SC and IV biopharmaceuticals to identify LVSC agents (Figure 1).
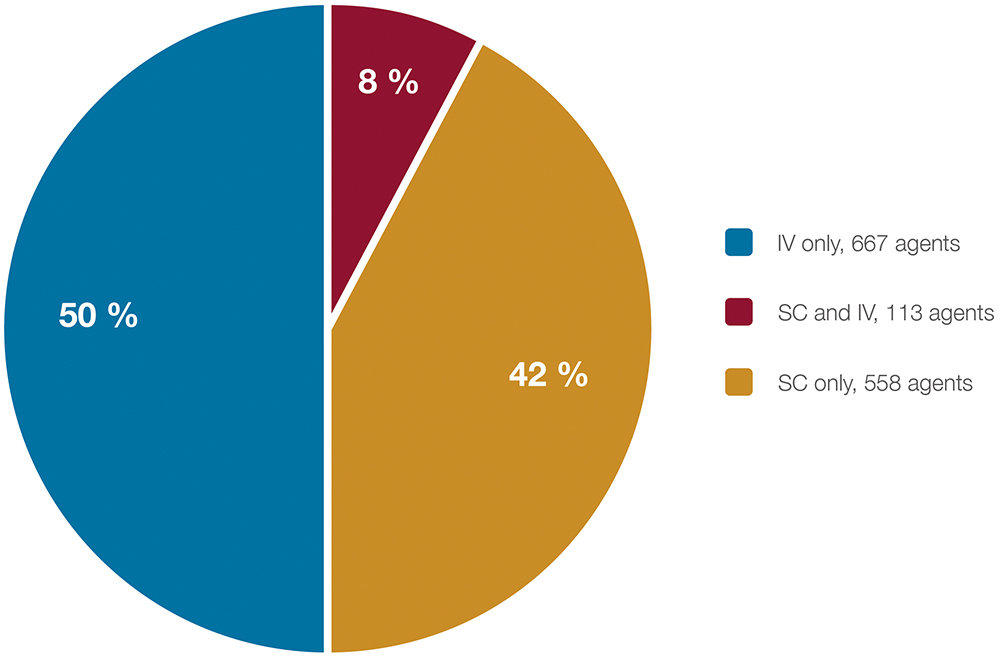
Figure 1: Route of administration and total number of approved and clinical-stage agents (N = 1,338) that comprise the initial sample at the outset of the search for LVSC biopharmaceuticals.
The study identified 182 LVSCs – predominantly monoclonal or bispecific antibodies – representing approximately 15% of all IV and SC biopharmaceuticals. These agents target both cancer and a range of chronic non-cancer conditions, including autoimmune, neurological and cardiovascular diseases. Figure 2 illustrates the distribution of these LVSCs by therapeutic category.
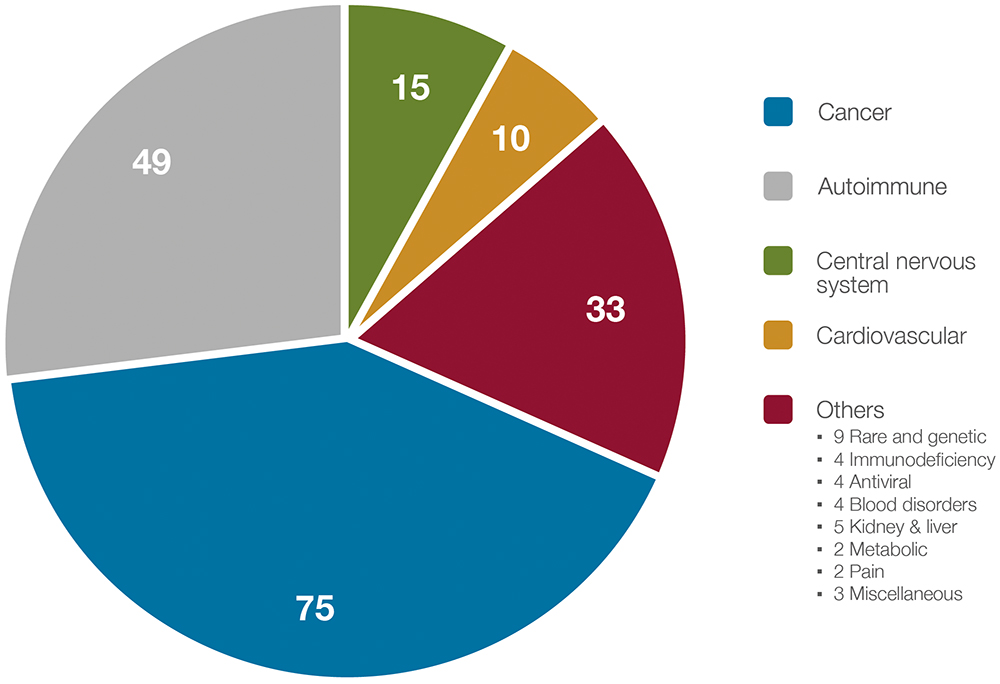
Figure 2: Therapeutic areas for approved and clinical-stage anti-cancer and non-cancer LVSCs (N = 182).
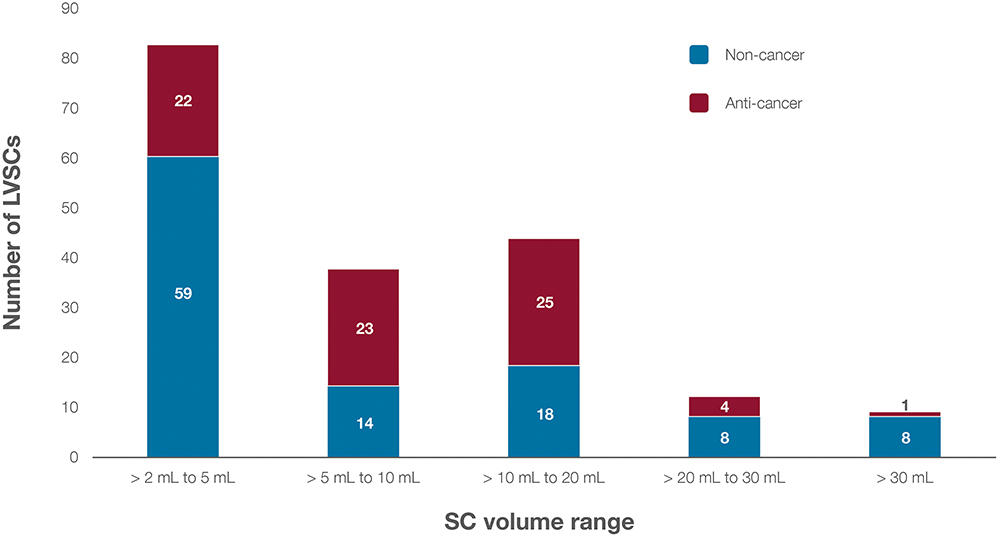
Figure 3: Distribution of LVSCs (N = 182) by dose-volume range and therapeutic category (anti-cancer versus non-cancer).
When analysing the dose-volume ranges of these LVSCs, the distribution highlights distinct trends by clinical development stage. Figure 3 shows the distribution of these LVSCs by dose volume and therapeutic category, with a notable concentration in oncology and autoimmune diseases. Clinical-stage LVSCs tend to have lower dose volumes, with 63% of clinical-stage agents falling into the 2–5 mL range compared with 48% of approved LVSCs in the same range. Conversely, IV-to-SC candidates often require higher volumes, with 76% of these agents requiring doses >5 mL. Figure 4 depicts dose-volume ranges across clinical development stages.
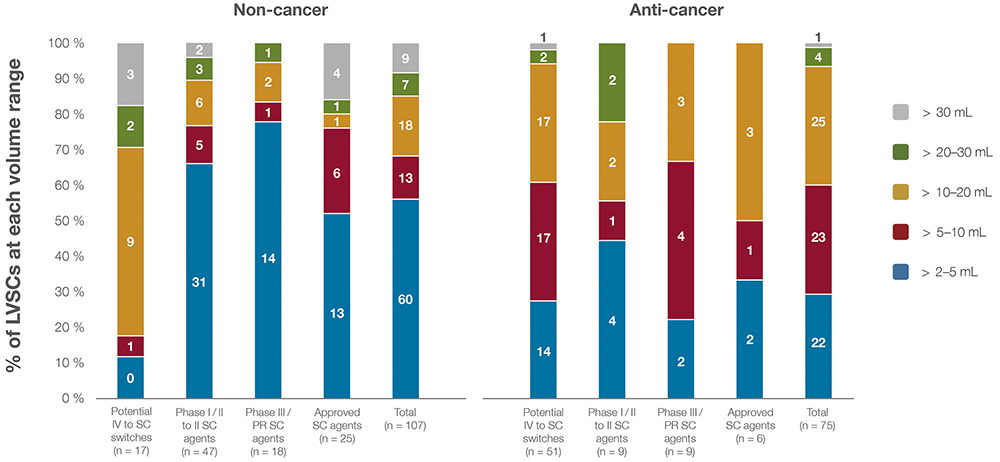
Figure 4: Dose-volume ranges for all IV-to-SC switching candidates, SC clinical-stage assets and approved LVSCs (N = 182) for non-cancer and anti-cancer indications across various stages of clinical testing. The number of LVSCs in each volume range is indicated on the bar chart.
The study also discusses LVSC dose-volume ranges by clinical development stage, LVSC modality types, biological targets, therapeutic areas and dosing intervals, providing a comprehensive overview of the LVSC landscape. These insights form the basis for designing future delivery systems tailored to the diverse needs of new and existing biopharmaceuticals.
CHALLENGES AND OPPORTUNITIES FOR LVSC DEVICE DELIVERY
LVSCs span all SC injection volume tiers, with most therapies falling within the 2–20 mL range. The most common tier is 2–5 mL, with 68 out of 81 LVSCs in this category being ≤4 mL. Handheld injections of 2 mL are widely accepted, and evidence suggests that handheld devices for doses well above 2 mL are possible. However, one of the big questions currently under investigation is whether rapid handheld SC injections can be extended to volumes as high as 5 mL, if not 10 mL. This extension would significantly expand the scope of handheld autoinjectors in the market, enabling broader self-administration options.
For the self-administration of volumes between 4 and 10 mL, manual injection remains a key delivery method, particularly when combined with permeation enhancers. This approach temporarily modifies the extracellular matrix, facilitating the injection of larger SC volumes. Such manual injections, typically performed by healthcare providers (HCPs) for up to 10 minutes, are already used for many large-volume agents. However, efforts are underway to shorten administration times and explore automation through handheld autoinjectors, which would enable patients to self-inject, even at these larger dose volumes.
For volumes exceeding 10 mL, alternative delivery methods, such as OBIs and syringe pumps, become more important. OBIs, which are wearable injection devices, can facilitate SC administration of larger doses either in a clinical setting or at home, offering significant convenience for patients requiring regular treatments. These devices are particularly suited for chronic conditions requiring consistent administration rates, such as autoimmune disorders. Syringe pumps are practical for patients with limited IV access and can deliver consistent doses over extended periods. At volumes exceeding 30 mL, manual injections and multiple OBIs may become impractical, making tethered SC infusion pumps a viable alternative. These pumps are already established in the market for therapies such as SC immunoglobulins, which often require high-volume administration.
“Another strategy to manage the challenges of large-volume SC delivery is to reduce the physical volume of injections by increasing drug concentration.”
Another strategy to manage the challenges of large-volume SC delivery is to reduce the physical volume of injections by increasing drug concentration. High concentration formulations (>200 mg/mL) enable smaller injection volumes, but these often lead to higher viscosities, which present additional challenges for device design. For such formulations, devices must employ advanced technologies, such as shorter and wider needles to reduce injection force or high-force autoinjectors capable of delivering viscous solutions without compromising user comfort.
The distinction between non-cancer and cancer LVSCs further highlights unique challenges and opportunities. Non-cancer LVSCs, such as those targeting asthma, inflammatory bowel disease and migraine, typically fall into the 2–5 mL range, making them well-suited for handheld autoinjectors designed for at-home self administration. However, some clinical stage non-cancer LVSCs require volumes exceeding 5 mL, necessitating OBIs or tethered pumps for home or outpatient use. For conditions such as Alzheimer’s disease, which involve significant unmet medical needs, the development of convenient SC delivery systems would not only enhance access to therapies but also improve overall patient care.
In contrast, most cancer LVSCs fall within the 5–20 mL range, with many requiring administration in a hospital setting by an HCP. These injections are typically delivered over 2–7 minutes, often in combination with hyaluronidase. While this approach is effective, there is a growing interest in shifting some cancer LVSC treatments to outpatient clinics, or even home settings. This transition will require more user-friendly delivery technologies capable of handling larger volumes without compromising efficacy or patient safety. Devices such as OBIs, syringe pumps and even advanced handheld autoinjectors are expected to play a crucial role in facilitating this transition.
However, several barriers remain for home-based self-injection of cancer LVSCs. For instance, patients undergoing combination therapy with IV chemotherapy or radiotherapy often visit infusion centres, limiting the added value of at-home SC administration. Despite this, there is potential for certain cancer patients to self-administer their treatments at home, either independently or with assistance from caregivers or HCPs. Clinical studies are currently investigating the feasibility of at-home administration for various anti-cancer agents and new delivery technologies are being designed to address these scenarios.
Another critical factor in LVSC delivery is dosing frequency. There has been a notable shift towards less frequent, more patient-friendly dosing intervals, such as every four weeks. This trend is particularly prominent in non-cancer LVSCs, where extended dosing intervals are associated with improved adherence and patient satisfaction. For cancer LVSCs, dosing schedules often align with IV chemotherapy or radiotherapy regimens, typically every three weeks. However, some therapies are moving towards longer intervals, such as every four or six weeks. These extended intervals necessitate larger SC doses, creating additional challenges for device design.
“Innovative delivery solutions that accommodate large volumes, high viscosities and frequent dosing schedules will be critical to meeting patient needs.”
Recent advancements in drug development are also driving the growth of LVSC biopharmaceuticals. Anti-cancer agents, for example, often require high systemic doses (>300 mg) to offset reduced SC bioavailability or to effectively target difficult-to-reach tumour sites. Similarly, autoimmune and neurological conditions frequently demand large SC doses due to high circulating levels of endogenous targets or rapid protein turnover. In these cases, innovative delivery solutions that accommodate large volumes, high viscosities and frequent dosing schedules will be critical to meeting patient needs.
POTENTIAL FOR IV-TO-SC TRANSITION AND IMPLICATIONS FOR DEVICE DESIGN
Ypsomed is addressing the growing demand for SC injections of high-dose therapeutics – the YpsoMate 2.25 and YpsoMate 2.25 Pro offer a reliable platform for <2 mL SC injections, featuring a simple two-step automatic process. To accommodate higher-viscosity formulations, the YpsoMate product platform range can be configured with an 8 mm needle, which reduces injection force through a thinner wall for rapid yet comfortable delivery.
Expanding this platform, the YpsoMate 5.5 supports volumes of 2–5.5 mL. Based on the proven YpsoMate 2.25 Pro technology, it employs a constant force drive mechanism to reproducibly inject viscous, large-volume formulations – essential for many high-dose therapies that conventional compression springs cannot handle.
Figure 5: Ypsomed solutions for large volumes and high viscosities. Pictured from left to right: YpsoMate 2.25 with 8 mm needle configuration, YpsoMate 2.25 Pro with 8 mm needle configuration, YpsoMate 5.5 and YpsoDose.
For larger doses, the YpsoDose patch injector manages payloads <1,000 mg in 5–10 mL volumes. This modular, customisable platform uses Ypsomed’s expertise in prefilled pen injectors and autoinjectors, accelerating time to clinic while minimising pharma partners’ upfront risks. It provides an effective solution for delivering high-dose medications requiring larger volumes or higher viscosities. Figures 5 and 6 illustrate Ypsomed’s portfolio of device solutions for LVSC administration and their relationship to the viscosities and volumes supported.
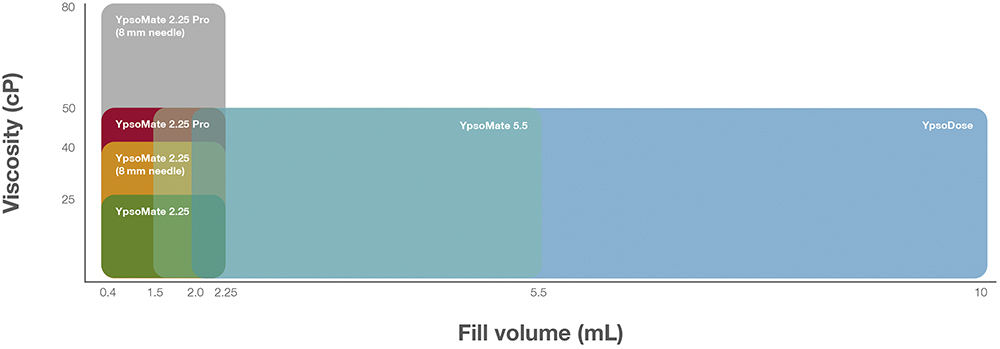
Figure 6: Viscosity (cP) versus volume (mL) for YpsoMate (YM) 2.25 autoinjectors, including models with the 8 mm needle configuration, YpsoMate 5.5 and YpsoDose.
THE FUTURE OF LARGE-VOLUME SC BIOPHARMACEUTICALS
The study highlights key trends in LVSC biopharmaceuticals, including the increasing focus on SC delivery for both cancer and chronic diseases, the shift towards patient-friendly dosing intervals and the evolving need for innovative delivery devices. These insights underscore the growing demand for technologies capable of managing high-dose therapies, including high viscosities, large volumes and diverse drug formulations for different therapeutic needs.
Together, Ypsomed’s comprehensive portfolio of innovative device solutions addresses the full spectrum of large-volume and high-viscosity SC delivery needs. With platforms designed to streamline the delivery of high-dose therapies, Ypsomed’s self-injection devices ensure scalability, patient comfort and ease of use. Designed for fast time-to-market and global success, these solutions support the transition from IV to SC administration, making self-injection accessible to a wide range of patient populations and therapeutic applications. As the field of LVSC biopharmaceuticals continues to expand, Ypsomed remains committed to partnering with pharmaceutical innovators to drive the future of high-dose biopharmaceutical delivery.
REFERENCE
- Green P, Schneider A, Lange J, “Navigating large-volume subcutaneous injections of biopharmaceuticals: a systematic review of clinical pipelines and approved products”. MABS, 2024, Vol 16(1), 2402713, pp 1–19.

