Citation: Barichello E, Valbonesi C, “A New Dimension: Balancing Biologic Limitations and Patient Perceptions for an Enhanced Injection Experience”. ONdrugDelivery, Issue 166 (Oct 2024), pp 26–30.
Enrico Barichello and Carlo Valbonesi discuss the need to harmonise the formulation parameters with patient convenience and comfort for injections, and demonstrate how Stevanato Group has solved this issue through innovation in injector components.
As the development of injectable drugs has continued to push boundaries, new challenges have arisen when it comes to their delivery. In each case, the specific requirements for administering an injection must be harmonised with the particular physico-chemical parameters of the formulation if, together, they are to deliver the efficacy and safety required, while also supporting convenience, increasing comfort and promoting adherence.
These benefits are felt keenly by the increasing number of patients affected by chronic conditions. It is estimated that non-communicable diseases represent more than half of the global disease burden, with a third of the global adult population suffering from multiple chronic conditions.1 Given that regular subcutaneous (SC) injections are needed for the ongoing management of many of these conditions, any opportunity to alleviate the burden of injections is likely to be welcomed, particularly as a large proportion of patients associate the experience with discomfort, anxiety and even fear to varying degrees.2 While subjective, such feelings are, of course, entirely valid and must be taken seriously to avoid the risk of treatment avoidance or abandonment.
“While the transition to an autoinjector offers greater freedom and control to patients, these devices are limited in terms of the maximum forces they can apply to power an injection.”
Potential solutions to this challenge include reducing injection frequency to minimise visits to clinical settings or enabling patients to self-administer their medications, possibly with the support of an autoinjector. These options bring several critical variables into play, however. For example, if the problem of injection frequency is solved by increasing the concentration of the dose, this can lead to an increase in the viscosity of the formulation. This could potentially exacerbate an existing problem for biologics, where viscosities are often already high, as it could lead to an extension of the injection time, negatively impacting the patient experience.
Furthermore, while the transition to an autoinjector offers greater freedom and control to patients, these devices are limited in terms of the maximum forces they can apply to power an injection, presenting a mechanical challenge for the delivery of more viscous drug products.
Increasing the physical size of the needle has the potential to alleviate the need for a higher injection force when delivering higher-viscosity formulations, but larger needle geometries have been associated with increased pain.3 Meanwhile, strategies to reduce injection frequency by increasing drug volume can lead to an unwanted increase in injection time or may not even be possible with an autoinjector, which typically delivers doses of ≤2 mL.
Stevanato Group has shown how this conundrum can be solved through innovation in injector components. This takes the form of the company’s 12.7 mm special thin-wall (sTW) needle, which maintains the traditional 12.7 mm needle length but features an optimised internal diameter to reduce the forces required when injecting high-viscosity formulations compared with a typical thin-wall (TW) needle. Crucially, this is achieved without increasing the external dimensions of the needle, which avoids increased pain.
Stevanato Group has also developed an 8 mm sTW needle, which features the same expanded internal diameter but with a reduced length. This further reduces the frictive potential of the needle, meaning the required injection force is further reduced. At the same time, the length of the needle has been designed to minimise the risk of accidental intramuscular delivery, which is a notable risk in patients such as children where the SC layer is thinner. With a shorter length and expanded internal diameter, the specifications of the needle enable the safe and effective delivery of high-viscosity formulations, as well as laboratory evaluation of break-loose extrusion has been undertaken to confirm the lower injection force.
A component’s performance can only be truly verified when it is assessed in real-world situations. Fundamentally, syringes are tools to be used by an individual and, while the technical parameters of each component must conform to precise physical specifications, they will, ultimately, be judged by the healthcare professionals (HCPs) and patients who directly interact with them – only they can decide whether a design truly delivers the intended benefits.
To answer this question, Stevanato Group conducted a rigorous human factors study comparing the performance of the new 8 mm sTW needle against the longer 12.7 mm sTW needle and the 12.7 mm TW needle, which features a slightly narrower internal diameter. The study aimed to explore the perceptions of four stakeholder groups who would be tasked with performing injections: HCPs, caregivers (CGs), experienced self-injecting patients (EXAPs) and inexperienced self-injecting patients (NAPs). Their assessments recorded the force required to push the plunger rod during injection, their confidence in the experience, whether they could perceive any difference between the needles and which needle they preferred.
The usability study paired the three needle variants with 2.25 mL syringes at injection viscosities of 10 and 30 cP. A total of 43 participants were enrolled in the study across the four different stakeholder groups, each of which carried out simulated injections on an injection pad using all syringe samples, which were presented in a randomised order. Any errors or difficulties were noted independently, while the participants self-reported different attributes of the injection experience according to seven items on a Likert scale – each item measuring how an attribute was experienced. Feedback was provided on general ease of use as well as perceptions of ease relating to insertion of the needle, holding the syringe while performing the injection and pushing the plunger. In addition, participants were asked to record their levels of anxiety and confidence in using the needles. Preferences across the syringe types and viscosities were indicated through the allocation of counters (Table 1).
| Viscosity Group |
Device | Total Assigned Counters | ||||
| EXAP | NAP | CG | HCP | Total | ||
| 10cP | A – 8 mm sTW | 30 | 42 | 24 | 22 | 118 |
| B – 12.7 mm sTW | 18 | 29 | 23 | 30 | 100 | |
| C – 12.7 mm TW | 6 | 7 | 13 | 14 | 40 | |
| 30cP | D – 8 mm sTW | 31 | 41 | 23.5 | 19 | 114.5 |
| E – 12.7 mm sTW | 19 | 17 | 22.5 | 31.5 | 90 | |
| F – 12.7 mm TW | 4 | 2 | 8 | 9.5 | 23.5 | |
Table 1: Total preference scores for the 10 and 30 cP viscosity injections.
OVERALL PREFERENCE
In terms of overall preference, the 8 mm sTW needle was rated highest for both viscosities based on the total combined score of all participants in the four test groups. Conversely, the 12.7 mm TW needle was the least preferred among all users, with universal agreement that the push force required was comparatively higher for this syringe variant.
Preference for the 8 mm sTW needle was particularly strong among the two patient groups, who noted the benefits of a lower push force and the shorter needle length, which was expected to enable a less painful self-injection. These two attributes together determined a higher confidence and acceptance for the 8 mm sTW needle among these participants.
The CGs ranked the 8 mm sTW and the 12.7 mm sTW options almost equally; their preference was split between the two needle lengths, with a primary focus on ease of push.
For the HCPs, meanwhile, the 12.7 mm sTW needle scored the highest preference rating. In feedback, this group cited the benefit of reduced injection force in line with other stakeholder groups, however, they also found the familiarity of the standard needle length appealing. This preference was strongly linked to the fact that they regularly use 12.7 mm needles during the course of their work and training. The HCPs acknowledged that their position could change on receipt of training regarding the use and benefits of a shorter 8 mm needle option, including being provided with the necessary assurances that the SC tissue can be reached in all types of patients despite the shorter length of needle.
EASE OF PUSH
Both patient groups had a more positive rating for the 8 mm sTW versus the 12.7 mm sTW at both viscosities for ease of push. Interestingly, the HCPs and GGs were more positive regarding “ease of push” for the 8 mm sTW than for the 12.7 mm sTW with the 30 cP (higher viscosity) injections, although this was not the case for the lower viscosity (10 cP).
At low viscosity, the ease of push between the 8 and 12.7 mm sTWs appeared almost equal for HCPs and CGs, with a more familiar needle (12.7 mm) contributing to a more positive rating, especially for CGs. However, as viscosity increases, the benefit of a shorter needle in reducing the required push force becomes more apparent, even to those who did not prefer the 8 mm needle at lower viscosities (Table 2).
| 10 cP | 30 cP | ||||||
| A – 8 mm sTW | B – 12.7 mm sTW | C – 12.7 mm TW | D – 8 mm sTW | E – 12.7 mm sTW | F – 12.7 mm TW | ||
| EXAP | Average | 5.9 | 5.7 | 3.8 | 4.8 | 3.7 | 2.6 |
| Median | 6 | 6 | 4 | 5 | 4 | 3 | |
| NAP | Average | 5.2 | 4.3 | 3.2 | 3.2 | 2.5 | 1.5 |
| Median | 5 | 5 | 3 | 3 | 3 | 1 | |
| CG | Average | 5.3 | 5.6 | 4.4 | 3.6 | 3.5 | 2.3 |
| Median | 5 | 6 | 4 | 4 | 3 | 2 | |
| HCP | Average | 5.6 | 5.8 | 4.8 | 5.1 | 4.5 | 3.6 |
| Median | 6 | 6 | 5 | 5 | 5 | 4 | |
Likert-item scoring range: 1=Very strongly disagree 2=Strongly disagree 3=Disagree 4=Neither disagree nor agree 5=Agree 6=Strongly agree 7=Very strongly agree
Table 2: Ease of push scores.
In summary, the results of the study show that the expanded internal diameter of sTW needles are preferred by patients, CGs and HCPs alike, thanks to the lower injection forces required. Moreover, the shorter 8 mm version of the sTW needle is preferred by patients, particularly if they lack experience administering injections, as it is linked to a perception of reduced pain during self-administration. For CGs, while no real preference was shown in relation to needle length, there was a clear preference for options with increased internal diameter where injection force was reduced. And while HCPs showed specific preference for longer needles, they are open to the benefits that shorter needles can bring.
While adjusting to the idea of a shorter needle can be supported through training, communication and the familiarity that comes with time, Stevanato Group has ensured that no significant adjustments are necessary from a manufacturing and product-handling perspective for such a transition. The 8 mm sTW needle, samples of which are available now, is packaged with the same rigid needle shield (RNS) as the 12.7 mm version, with continuity achieved through recalibration of the interior profile of the RNS. It is also compatible with both Stevanato Group’s Alba® and Nexa® glass primary packaging lines, which not only meet the mechanical and cosmetic demands of high-performance applications but also offer a complete containment solution through the use of cross-linked coating technology and elastomer components formulated to protect sensitive biologics.
“Given the burden of injections in terms of their frequency, as well as the force and time involved, there is a clear need to optimise this process.”
Given the burden of injections in terms of their frequency, as well as the force and time involved, there is a clear need to optimise this process for speed and ease of use to enhance the patient experience and support long-term adherence to treatment regimens for patients with chronic conditions. In reality, that means accommodating fewer, less painful injections of more viscous drug products, ideally via autoinjector devices, to support an improved patient experience. Already, the development of a sTW needle allows many of these benefits to be achieved in 12.7 mm form, and the introduction of the new 8 mm sTW variant extends these capabilities further for accuracy and ease of use in SC injections.
However, the objective advantages provided by the specification of these needles must be assessed in the subjective light of experience. Through analysis, Stevanato Group has been able to demonstrate this point, showing how injection perceptions can differ, not just in line with different needle types and injection viscosities but also according to the feelings, knowledge and expectations of the person administering the injection. For patients, when the internal diameter and the length of the needle are optimised to make this experience easier and quicker, perceptions around pain decrease and there is an increased level of acceptance.
To the untrained eye, it might be easy to assume that all injections are essentially the same. With every dose delivered, the essential elements of needle, cartridge and plunger combine in a consistent action and common patient experience. However, as discussed here, the delivery of injectable drug products in the real world must bring together precision-designed components engineered to critical tolerances. Only then will the most critical stakeholder – the patient – feel confident in both the immediate moment of the injection and in the ongoing benefits of their therapy.
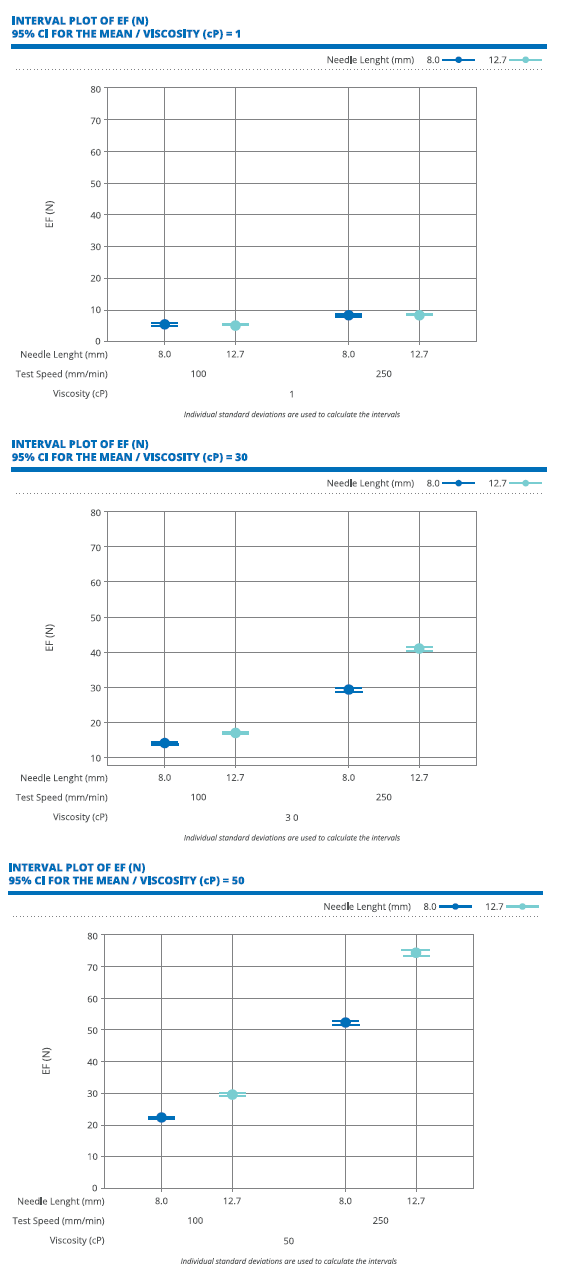
Figure 1: In samples filled with highly viscous glycerol-based solutions (30 and 50 cP), the syringes assembled with the 8 mm sTW needle resulted in lower EF than the syringes assembled with the 12.7 mm sTW needle.
“Stevanato Group has conducted internal testing at the company’s Technology Excellence Centers (TEC) to assess the effect of the needle’s exposed length.”
EFFECT OF THE EXPOSED NEEDLE LENGTH
In addition to the human factors study, Stevanato Group has conducted internal testing at the company’s Technology Excellence Centers (TEC) to assess the effect of the needle’s exposed length.
Consistent with the human factors study, the company’s US TEC tested two 2.25 mL syringes: the first with an 8 mm exposed needle and the latter with a 12.7 mm exposed needle, both sTW.
The syringes were filled with water and glycerol-based solutions characterised by different target viscosities (1, 30 and 50 cP) and then tested via break-loose extrusion testing to assess the extrusion force (EF) at two different test speeds (100 and 250 mm/min).
As shown in Figure 1, the results show that, for samples filled with the highly viscous glycerol-based solutions (30 and 50 cP), the syringes assembled with the 8 mm sTW needle resulted in a lower EF than the syringes assembled with the 12.7 mm sTW needle. This difference was magnified in the high-speed (250 mm/min) tests. No difference was observed with the water-filled samples.
CONCLUSION
Overall, optimising injection systems to reduce the injection frequency, associated pain and required effort is essential for enhancing the patient experience and adherence, especially when chronic conditions. Stevanato Group’s innovations in needle design demonstrate significant progress in achieving these goals, with real-world usability studies validating the benefits perceived by patients and HCPs.
REFERENCES
- “This is the biggest challenge to our health”. World Economic Forum, Dec 7, 2017.
- Alsbrooks K, Hoerauf K, “Prevalence, causes, impacts, and management of needle phobia: An international survey of a general adult population”. PLoS One, 2022, Vol 17(11), article: e0276814.
- Gill HS, Prausnitz MR, “Does Needle Size Matter?”. J Diabetes Sci Technol, 2007, Vol 1(5), pp 725–729.
Previous article
THE PERFORMANCE OF EUROJECT – A BFS-BASED INJECTION DEVICENext article
COMPANY SHOWCASE: PCI PHARMA SERVICES
