Citation: Khanolkar A, “A New Mindset for Combination Product Development”. ONdrugDelivery, Issue 125 (Oct 2021), pp 28–33.
Asmita Khanolkar outlines the latest trends in combination product development to overcome some of the current challenges of high dose/viscosity/volume delivery.
Based on the learnings from the covid-19 pandemic, it is time to emphasise the changing mindset towards a forward-looking design and development process for combination products. The new outlook for the pharmaceutical industry is very different from the one we knew prior to the pandemic. The new normal encompasses rapid development of treatments and new regulatory pathways to support the urgency towards faster times to clinic.
Treatments are now administered outside traditional hospital care settings, including clinical studies conducted at home. We have seen a dramatic shift from a one-size-fits-all approach towards rising personalised medicine. Novel therapies considered too complex and complicated previously are now available in the hands of patients, and there is an unmet need for enabling device technology that can handle the challenging formulations. Finally, global digital transformation is modernising the overall healthcare experience.
Moving forward, as we balance the time to market and risk for novel therapies, we can anticipate several trends, including: a changing mindset in areas of combination device development focused on enabling device design for challenging applications for optimising delivery; patient-centric interfaces for self-administration to eliminate user errors; and integrated drug-device development iteration cycles to minimise any risks for clinical outcomes. The latter highlights a technological paradigm shift and focus on developing combination products at pandemic speed.
COVID-19 IMPACT ON THE DRUG DEVELOPMENT PROCESS
The drug development process can be broadly divided into three segments – drug discovery through preclinical, clinical evaluation through approvals and finally commercial launch. Each of these segments were significantly impacted by the pandemic but in different ways (Figure 1).
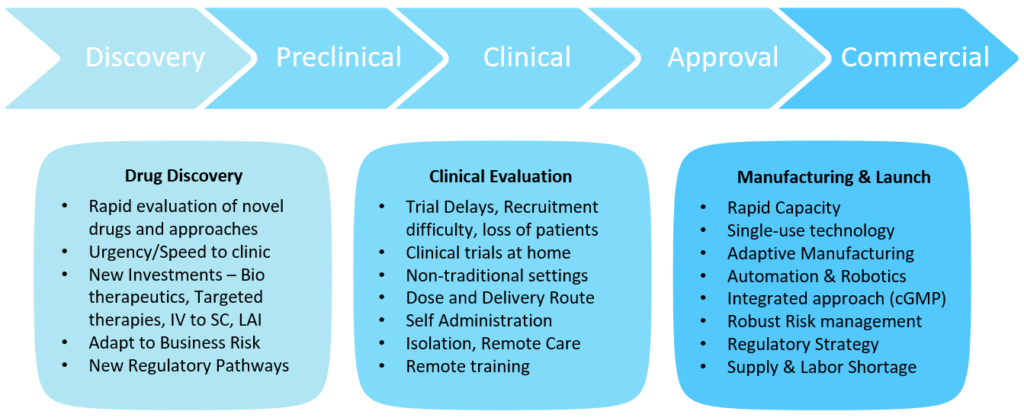
Figure 1: The impact of covid-19 on the drug development process.
“With the pandemic and the difficulty of getting patients to a hospital, the trend has moved towards subcutaneous (SC) delivery and increasing the timing between treatments.”
The drug discovery segment saw the urgency of getting therapies to clinic faster than ever with patients in need from both the pandemic infectious disease itself and the amplification of struggles with chronic and crisis diseases created by the pandemic. Previously for parenterals, an intravenous (IV) version would be considered for first release and the fastest path to patients. With the pandemic and the difficulty of getting patients to a hospital, the trend has moved towards subcutaneous (SC) delivery and increasing the timing between treatments – resulting in more challenging formulations for higher concentration, larger volumes and long-acting injectables (LAIs). New faster regulatory pathways for approvals for unmet needs further justified rapid evaluation of novel therapies and faster parallel approaches, posing multiple paths towards faster time, to clinic but, at the same time, accepting more business and investment risks. The clinical evaluation segment saw tremendous delays, difficulties in recruitment and loss of patients. We saw clinical trials move to non-traditional home settings from hospitals. Patients were not willing to go to hospitals during the pandemic and hospitals were overwhelmed with the pandemic response. It became apparent that clinical trials had to be decentralised from the hospital to home. This brought tremendous patient logistics and clinical trial supply management challenges.
The commercial launch segment saw the need for rapid capacity increases while supply shortages and labour resource management made it difficult to deliver the product. Single-use technology, adaptive manufacturing and automation trends were implemented to overcome some of these challenges, along with implementation of robust risk management procedures.
“The impact of covid-19 on the drug development process poses and necessitates a significant paradigm shift for combination products.”
SIGNIFICANT PARADIGM SHIFT FOR COMBINATION PRODUCT DEVELOPMENT
The impact of covid-19 on the drug development process poses and necessitates a significant paradigm shift for combination products. Rapid development of therapies that took years has now been reduced to months. This necessitates simultaneous development of drug and device. In addition, the complexity of formulations and challenging needs brought to light challenges of the legacy platform device technology that may not be suited for these novel applications. The risk increases especially for novel drug products that are complex molecules – and due to unknowns and uncertainties with the new delivery methods and large dosage. Other risk factors include patient tolerability and acceptability. The pharma industry is also looking for avenues for market differentiation. The success of the clinical outcome is dependent on the delivery optimisation in these situations and thus the realisation of an unmet need for an enabling design for early-stage characterisation and development of drug-device combinations for challenging therapies and unmet patient needs.
The shift of clinical trials to home has brought the focus on to self-administration for clinical trial supplies early on. The importance of optimal drug-device combinations in terms of user needs becomes critical for successful clinical outcomes. We are now looking at targeting designs for eliminating user errors completely. The healthcare professionals’ visits to patients at home have emphasised the need for streamlined devices. In addition, some of the novel targeted therapies – such as cancer treatment and biologic drugs – are costly. Administering them in hospitals over long periods of time can become unaffordable for patients. As a result, many targeted therapies and precision medicines are now being designed to be self-administered.
Finally, the complex formulations are not an exact fit to deliver with existing standard device technologies, and the device needs to be designed to deliver the specific formulation appropriately. In addition, formulations have to be optimised for delivery and for the therapy outcome. This results in iterative development cycles and the need for customisable processes, flexible lines and adaptive manufacturing technologies that can support the many facets of joint development of customised drug-device therapy for successful clinical outcomes (Figure 2).
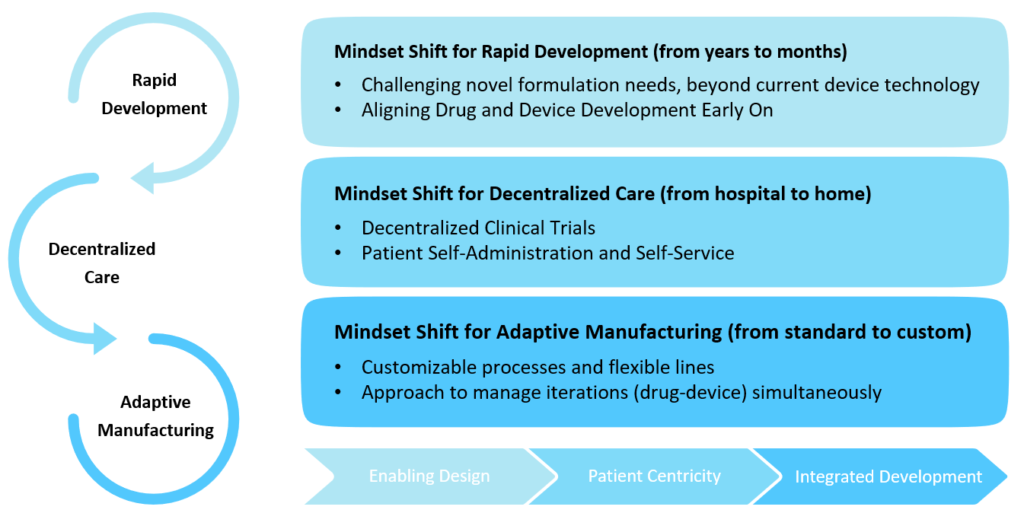
Figure 2: New mindset for combination product development.
CHALLENGING FORMULATIONS
Novel formulations involving LAIs and complex biotherapeutics pose challenges to the traditional drug delivery device platforms. In the case of biotherapeutics, we are dealing with complex high-molecular-weight molecules, such as monoclonal antibodies (mAbs) (Figure 3).

Figure 3: Complex molecules of biotherapeutics and biologics.
Additionally, these biologic molecules are fragile – and stability in the primary drug container is also important throughout the shelf life of the product. This requires clean systems free from any potential interactions, thus the need for silicone lubricant-free and tungsten-free systems. Trends of IV to SC delivery require high dose concentrations and large delivery volumes for administration subcutaneously. This requires a high-pressure system with reasonable and consistent delivery time. Previously, in glass syringes, patients have experienced variability of injection times – resulting in wet injections. In the case of biologic drugs, this can potentially cause an immunological response. Therefore, for such applications, traditional legacy primary packaging materials may not be suitable.
LAIs are formulated for extended times, supported over monthly, bi-monthly or even a three-month period. This is achieved through controlled release of the drug, typically through adjusting or reducing the solubility of the LAI. The approaches to developing slow-release formulations typically involve high-molecular-weight vehicles such as oil solutions, water-insoluble suspensions or crystalline polymeric barriers (Figure 4), which, in turn, increase the viscosity of the entire formulation and can also lead to non-Newtonian behaviour and increased sensitivity to environmental conditions. Delivering these via autoinjector can be further complicated by other characteristics of suspensions, such as settlement in storage, particle agglomeration or clogging. This puts great onus on the delivery mechanism for high-pressure systems to overcome and manage delivery requirements.

Figure 4: Formulation vehicles for LAIs.
The API is released from the carrier vehicle by either diffusion or degradation, both of which can occur most rapidly at the surface of the drug bolus. A bolus with a larger surface area will often release its API at a faster rate than one with a smaller surface area, reducing the effective duration of the dose. The shape of the bolus formed can therefore have a significant impact on the pharmacokinetic profile of the formulation. A spherical bolus is usually ideal as it reduces the release rate of the drug, potentially allowing a longer dosing interval. Delivery parameters such as injection speed can have an impact on the shape of the bolus and therefore the pharmacokinetic profile. The delivery parameters need to be optimised in conjunction with the formulation.

Figure 5: Device architecture for a flexible platform.
“It is evident that one size does not fit all, whether it be the personalised and targeted dosage regimen, control of needle depth due to physiological differences, emotional status of patients, population diversity or cost of the therapy.”
ONE SIZE DOES NOT FIT ALL
These discussions lead towards what the device architecture should look like going forward – it has to fulfil a lot of criteria, from the previously mentioned technical challenging needs of the formulations to the patient interface side of things when considering self-administration and self-service (Figure 5). It is evident that one size does not fit all, whether it be the personalised and targeted dosage regimen, control of needle depth due to physiological differences, emotional status of patients, population diversity or cost of the therapy. This brings us to the unmet need of an enabling design that can be customised internally to the challenging technical needs of the application and external customisation to patient touchpoints towards eliminating user errors and successful self-administration.
ENABLING DESIGN SOLUTION – ARQ-BIOS AUTOINJECTOR
Oval has developed a high-power, single-use autoinjector called the ArQ-Bios, which offers the ability to deliver high-viscosity or high-volume dose options for SC delivery in the same device. This allows flexibility for formulation development, early engagement with the device and reduced risk and time to market. Low-to-medium-viscosity formulations under 100 cP can be delivered up to 10 mL and high/ultra-high viscosities up to 10,000 cP can be delivered between 0.5 and 3 mL. Owning and manufacturing the primary drug container allows integrated devices to be designed for the needs.
Oval’s proprietary patented “cup seal and foil” technology is built around a high-pressure cyclic-olefin copolymer primary drug container (PDC). The PDC can safely tolerate significantly higher pressure than glass, allows stronger springs and enables devices to generate higher pressures than other market offerings. The high-pressure cup seal design overcomes the friction challenges of traditional rubber plunger seals. By decoupling the microbial and liquid seal barrier functions, conflicting requirements can be managed separately. The polyethylene piston component provides liquid seal with the stability to manage high pressures and sufficient lubricity to prevent excess glide forces. The induction-welded foil provides a microbial barrier and is a robust solution for high-viscosity delivery. The design aims for a superior patient experience and fewer wet injections due to highly consistent drug delivery times, independent of product age or manufacturing tolerances.
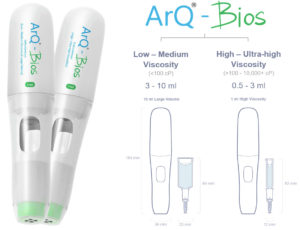
Figure 6: Enabling design – ArQ-Bios autoinjector technology.
The ArQ-Bios also incorporates a proprietary hydraulic valve release mechanism. The valve enables quiet and gentle activation of the device, even when the drug is pressurised at 300 bar. These unique features make ArQ-Bios an enabling technology for high-viscosity or high-volume applications for the demanding needs of LAIs or biotherapeutic SC delivery (Figure 6).
Digital and mathematical transformation techniques – such as predictive modelling and simulation – can be used to predict the delivery time of challenging formulations and predict the shear-dependent behaviour of LAIs and biologics. Using these models, a simulation can be created to predict autoinjector performance. The simulations can look at likely variations of injection times across millions of simulated devices constructed by random selection of different input variables. This can help with optimisation of the design (Figure 7).
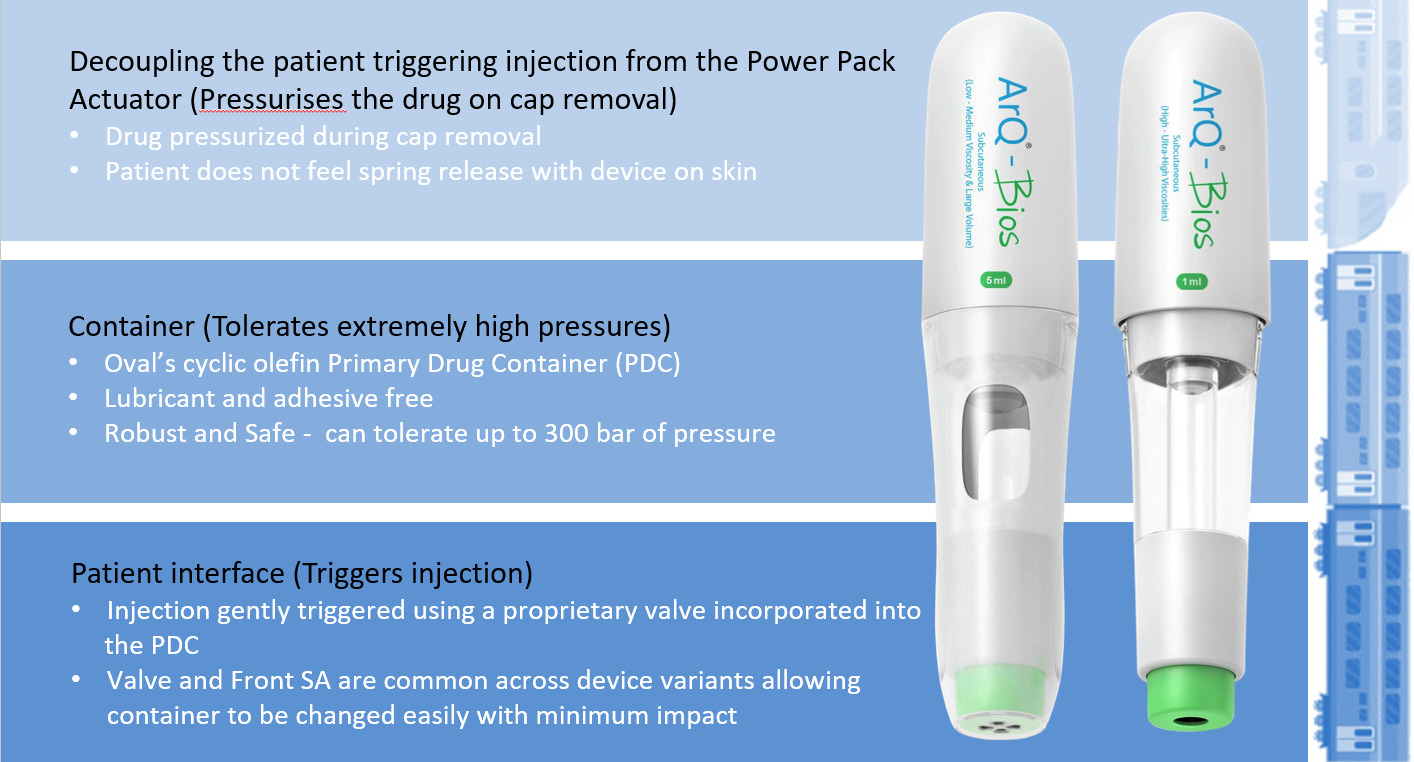
Figure 7: Patient interface focus – gentle self-administration.
INTEGRATED ADAPTIVE MANUFACTURING
The final consideration when discussing challenging formulations is the integrated drug-device-patient approach combining concurrent development, manufacturing and test cycles. This removes the fragmented approach and can substantially reduce time to clinic.
Especially in the case of LAI formulations, integrated studies are required throughout the development to optimise formulation parameters, API release and pharmacokinetics performance. Biotherapeutics have their own challenges of bioavailability optimisation from preclinical to clinical models, complex molecular structures and are typically administered in larger volumes and require optimisation of formulation and delivery for the route of administration. Oval’s ArQ-Bios platform offers a flexible platform for early-stage development for large-volume and high-viscosity biologic and LAI formulations. Engaging early with the device provides a unique solution to the challenge of delivering better solutions to patients (Figure 8).
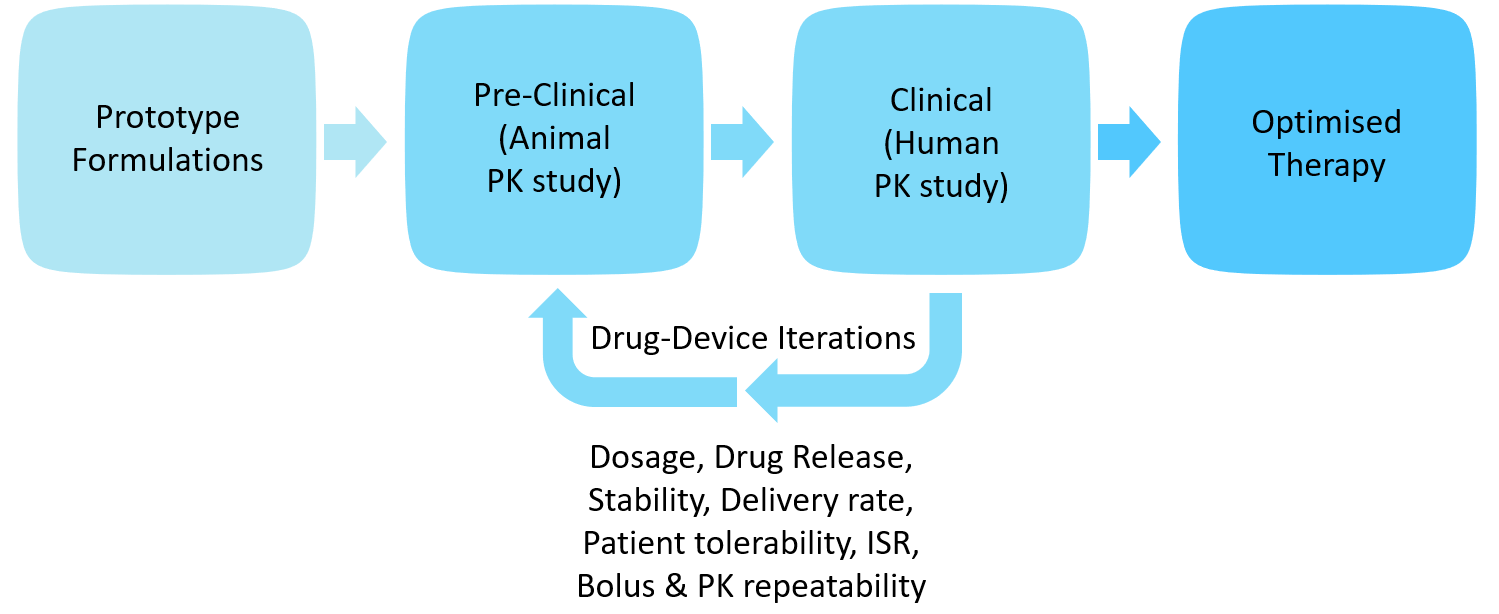
Figure 8: Iterative drug-device development.
In addition to the enabling design, adaptive manufacturing and flexible processes are needed for the optimisation iterative cycles. Starting from moulding and assembly, PDC moulding flexibility and design for manufacturing inputs are key manufacturing considerations. PDC moulding allows customised designs to be tailored to the needs of each drug. Tolerance control on the device assembly stack ensures repeatability and enhances device performance reliability. Fixturing and automation development early on help accelerate special processes industrialisation, including fill-finish and secondary packaging processes. Adaptive manufacturing, including customisable processes and flexible lines capable of GMP manufacturing, is key to the successful development through to commercial launch of novel therapies (Figure 9).

Figure 9: Integrated manufacturing from early research to launch.
In summary, this article has outlined the new mindset for combination product development to overcome some of the current challenges of high-dose/viscosity/ volume delivery with enabling device design, self-administered home treatment, customised patient interfaces eliminating user errors and methods of adaptive manufacturing to provide pathways for simultaneous drug-device development. As the pandemic continues, the focus on virtual care, longer times between hospital visits and the need for at-home care for chronic diseases will continue. This further translates into a growing need for self-administering biotherapeutics and LAI formulations subcutaneously. ArQ-Bios technology overcomes the limitations of existing legacy technology, providing enabling delivery technology for challenging formulations and better solutions for patients.

