To Issue 142
Citation: Khanolkar A, “Accelerating Novel Therapies to the Clinic – Custom Solutions”. ONdrugDelivery, Issue 142 (Feb 2023), pp 32–35.
Asmita Khanolkar discusses the key offerings necessary to accelerate “speed to clinic” for custom manufacturing solutions.
“Speed to clinic is more critical than ever for novel therapeutics due to potentially reduced market sizes.”
Pharmaceutical trends today are shifting towards targeted therapies, precision medicine and personalised treatment for smaller patient populations. With the growth of novel therapeutics, “speed to clinic” is more critical than ever. These novel therapeutics target a more specific indication, resulting in a smaller potential market. The overall revenue projection, along with available clinical study patients, are reduced – as seen in markets for oncology.
The formulations involved are more complex biotherapeutics, and large yet fragile molecules pose many unknowns and uncertainties throughout development. Bioavailability and immunogenicity are often not well understood, which means multiple iterations for therapy optimisation. In addition, delivery of these formulations is difficult, as they are often non-Newtonian or high viscosities and require custom high-pressure primary drug containers and devices. This requires flexibility to support adaptive and flexible sterile manufacturing towards an integrated approach for a path from development to small-batch manufacturing and commercialisation that can save time to clinic.
“Novel therapeutics often require customised delivery solutions for more targeted therapies.”
PHARMA SUPPLY CHAIN
The pharma supply chain still overwhelmingly represents the legacy needs of large-scale launches of blockbuster drugs with more “off-the-shelf” delivery options. In contrast, novel therapeutics often require customised delivery solutions for more targeted therapies. This gap or lack of alignment can increase the time required for development of novel therapeutics.
The unique combination of skills and expertise of the SMC Group (SMC Ltd, Oval Medical Technologies and Cambridge Pharma) aligns with the need for delivery customisation and results in reduced time to clinic for challenging novel therapeutics. With the combined offerings of SMC Ltd, Oval Medical and Cambridge Pharma, a portfolio of integrated services is provided – from the exploratory, preclinical stage through to clinical development and registration – to help accelerate the combination product development process from early stages onwards.
This includes formulation characterisation, drug product compounding, drug scale-up, innovative drug delivery device autoinjector platforms, animal testing, human factors studies, clinical sterile fill-finish and commercial manufacturing (Figure 1). Speed to clinic is more critical than ever for novel therapeutics due to potentially reduced market sizes, which impacts both return on investment and available clinical study patients – but, more importantly, the need to bring critical solutions to patients faster.
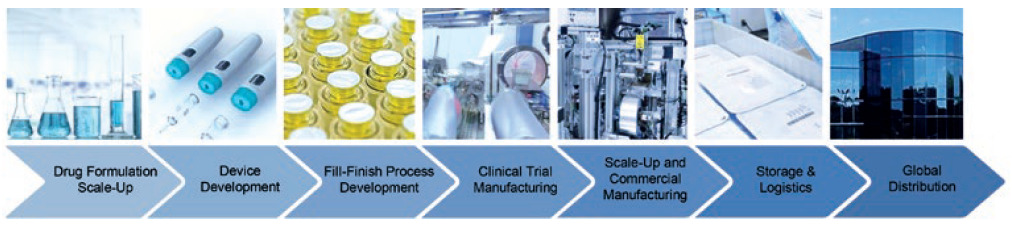
Figure 1: Integrated manufacturing from early research to commercial launch.
The following are the key offerings necessary to accelerate speed to clinic for custom manufacturing solutions:
- Vertically integrated development and manufacturing services to iterate quickly and cost effectively and avoid lengthy delays later in the development cycle.
- Enabling drug delivery solutions that can help early characterisation of formulations for optimised delivery of novel therapies.
- Small-batch manufacturing flexibility from clinical trials to commercial scale-up
- State-of-the art facility for GMP fill-finish with flexibility to handle legacy and customised delivery solutions for novel therapies.
- Specialised isolator equipment and filling experience to optimise fill-finish while maintaining container closure integrity (CCI) for challenging formulations.
- Experience providing solutions for challenging formulations including high viscosity, suspension and non-Newtonian fluids.
- Dedicated process development laboratory for process engineering solutions.
- Analytical laboratory solutions for test-method development and stability studies.
- Broad expertise across multiple facets of regulatory, clinical and commercial strategy.

Figure 2: Small-batch sterile manufacturing.
SMALL-BATCH MANUFACTURING
Small-batch manufacturing requires flexible filling processes and innovative equipment that can handle a range of primary drug containers, including prefilled syringes and custom-designed vials and cartridges. Fast, flexible fill-finish isolator-based filling suites including qualified person (QP) release set-up is needed for handling small batch sizes ranging from 100 units to 10,000 units.
In contrast to dedicated high-volume lines, small-batch manufacturing requires flexibility for changeovers. However, due to the complexity of these formulations, there may be conflicting requirements for specialised equipment. Thus, the need for innovation through single-use systems, automation, non-contact processing, robotic inspection and CCI testing (Figure 2).
Novel therapies, including biotherapeutics, have special manufacturing needs and may need special processes developed for drug compounding, scale-up and fill-finish processes. Different pump technologies and fill methodologies can help tailor filling suitable to the drug. Depending on the application, peristaltic pumps for use with sensitive products like biotherapeutics and contact-free or single-use applications, for example, versus rotary piston pumps and special heads, which are suited to highly viscous formulations. Separately, optimisation of aseptic drug filling, stability, storage and delivery profiles for custom devices while maintaining CCI requires significant process mapping steps. In addition, quality control (QC) and analytical test methods for biotherapeutics need significant development (Figure 3).

Figure 3: Speciality processes.
Adaptive manufacturing is a strategy that supports customisable processes and adjustments as the process is developed and unknowns of novel formulations are revealed. A true adaptive manufacturing process can enable adjustments to improve quality at each step as the process is developed from lab scale to GMP scale. For custom primary drug containers, mould design and tolerances can be adjusted to meet the needs of the drug. Flexible isolator configurations and controlled environment set-ups are essential for developing GMP similar processes from the beginning (Figure 4).
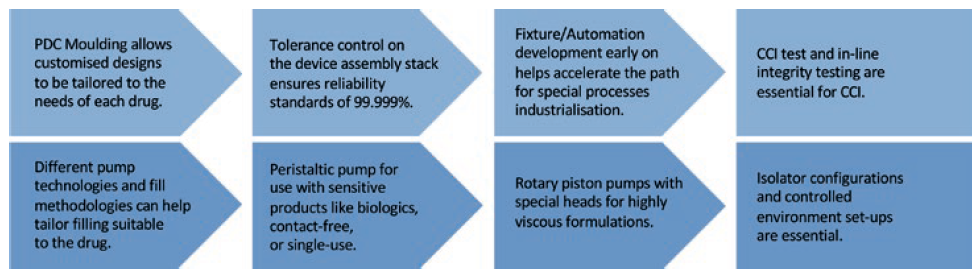
Figure 4: True adaptive manufacturing from start to finish.
“Continuing trends from hospital to home treatment have changed the clinical trials landscape.”
BROAD TECHNICAL EXPERTISE
Alongside the GMP manufacturing facility, process engineering laboratory and analytical laboratory capabilities allow the experienced scientific team to carry out process development work, analytical method transfer and validation, QC release and stability testing and, if required, QP certification to clinic. This type of process development work requires a holistic cross-functional approach and broad expertise looking at various facets of development and streamlining.
It is time consuming and challenging when engaging with multiple different suppliers and many items are missed at process hand-offs and interfaces. A single source partner with in-house expertise to support novel drug development from pre-clinical to commercialisation eliminates any fragmented approach and streamlines development. Process engineering and development information is leveraged for clinical production to eliminate risks early on, which reduces time to clinic (Figure 5).
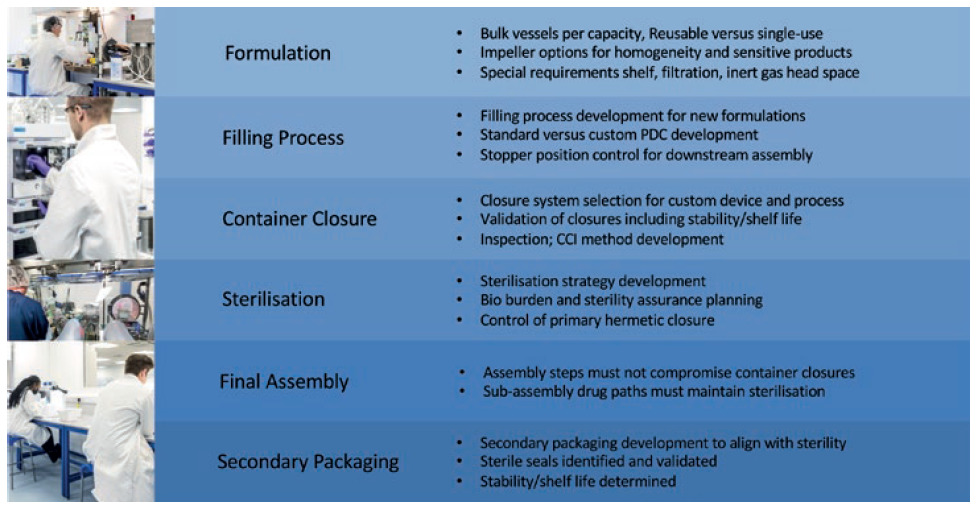
Figure 5: Streamlined process development interfaces.
CUSTOM IS THE NEW NORMAL
Novel formulations require characterisation for various aspects of drug filling, storage and delivery. Early characterisation at the start of a project eliminates lengthy delays at later stages in the programme (Figure 6).
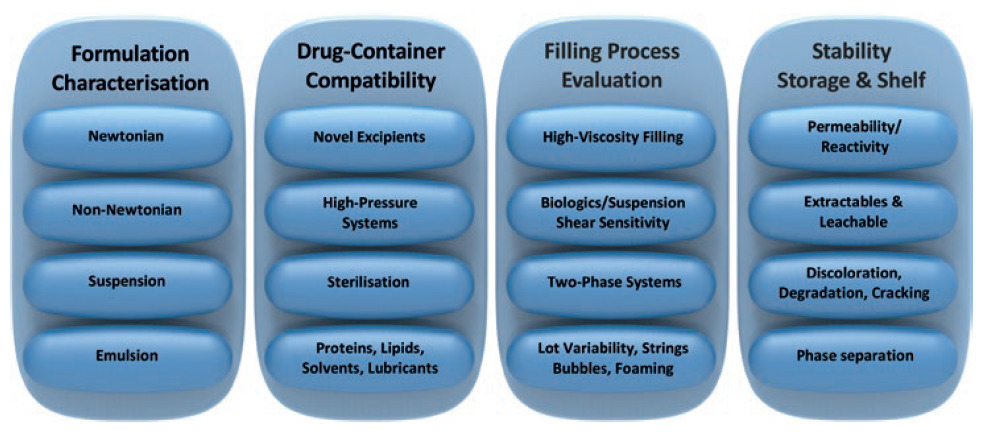
Figure 6: New drug formulation considerations.
STATE-OF-THE-ART FACILITY
The regulatory requirements landscape for GMP facilities is stringent and newer facilities have to meet the latest requirements for compliance as well as sustainability. Cambridge Pharma has state-of-the-art facilities with optimised material and people flow. Two filling suites are available for flexible small-batch manufacturing, including a clinical isolator line and a commercial semi-automated isolator line. Not only do the suites have innovative cleanroom control systems, enabling real-time monitoring and energy-efficient heating, ventilation and air conditioning systems, but they are also controlled separately so the suites can be operated independently.
NEW LANDSCAPE FOR CLINICAL TRIALS AND REGULATORY PATHWAY
Continuing trends from hospital to home treatment have changed the clinical trials landscape. This is driving the industry to focus more on the self-administration of therapies and the need for devices in clinical studies earlier in the cycle. The cost of therapy is also lower when delivered at home rather than in a hospital setting, which puts additional importance on speed to market. New regulatory pathways allow faster avenues for breakthrough therapies and emergency use. This means less time to commercially scale and a shorter development cycle.
In summary, as a single-source supply partner with small-batch manufacturing capabilities, SMC Ltd can substantially improve speed to market and de-risk programmes. A state-of-the-art facility with innovative equipment and systems provides the specialised systems needed for novel therapies. In addition to a GMP facility, a process development and analytical lab provides an adaptive manufacturing model and increases speed to clinic.

