Citation: Shaked T, “Adding Connectivity to Drug Delivery Devices”. ONdrugDelivery Magazine, Issue 98 (Jun 2019), pp 62-65.
Tsachi Shaked explores how – as the trend towards moving healthcare from the hospital to the home continues – connectivity in drug delivery devices may be changing from being the exception to the rule.
“39% of chronic condition patients prefer online consultations to face-to-face meetings.1”
The trend towards home-based care is being driven by healthcare providers as well as patients.For providers, it makes better economic sense to enable patients to be treated at home as it cuts down on costs and frees up both beds and the valuable time of healthcare professionals. For patients, it eliminates the inconvenience of having to make frequent trips to hospital or a clinic, saving time and money, whilst allowing treatments to be carried out in a more comfortable and familiar environment. Many patients with chronic conditions have indicated that they would prefer this option – a June 2017 industry insight report from Ericsson ConsumerLab showed that 39% of chronic condition patients prefer online consultations to face-to-face meetings.1
Progress in using connected drug delivery devices can currently be seen with inhalers and autoinjectors. In these use cases, responsibility for drug administration has fallen primarily on the patient, their family members or caregivers. But while this development has freed patients from visits to their healthcare professional or hospital, it has also reduced the opportunity for clinicians to monitor whether patients are administering drugs on schedule and in the right amount. Encouraging and supporting patient adherence continues to be a challenge and provides a further compelling case for adding connectivity to drug delivery devices.
PATIENT ADHERENCE
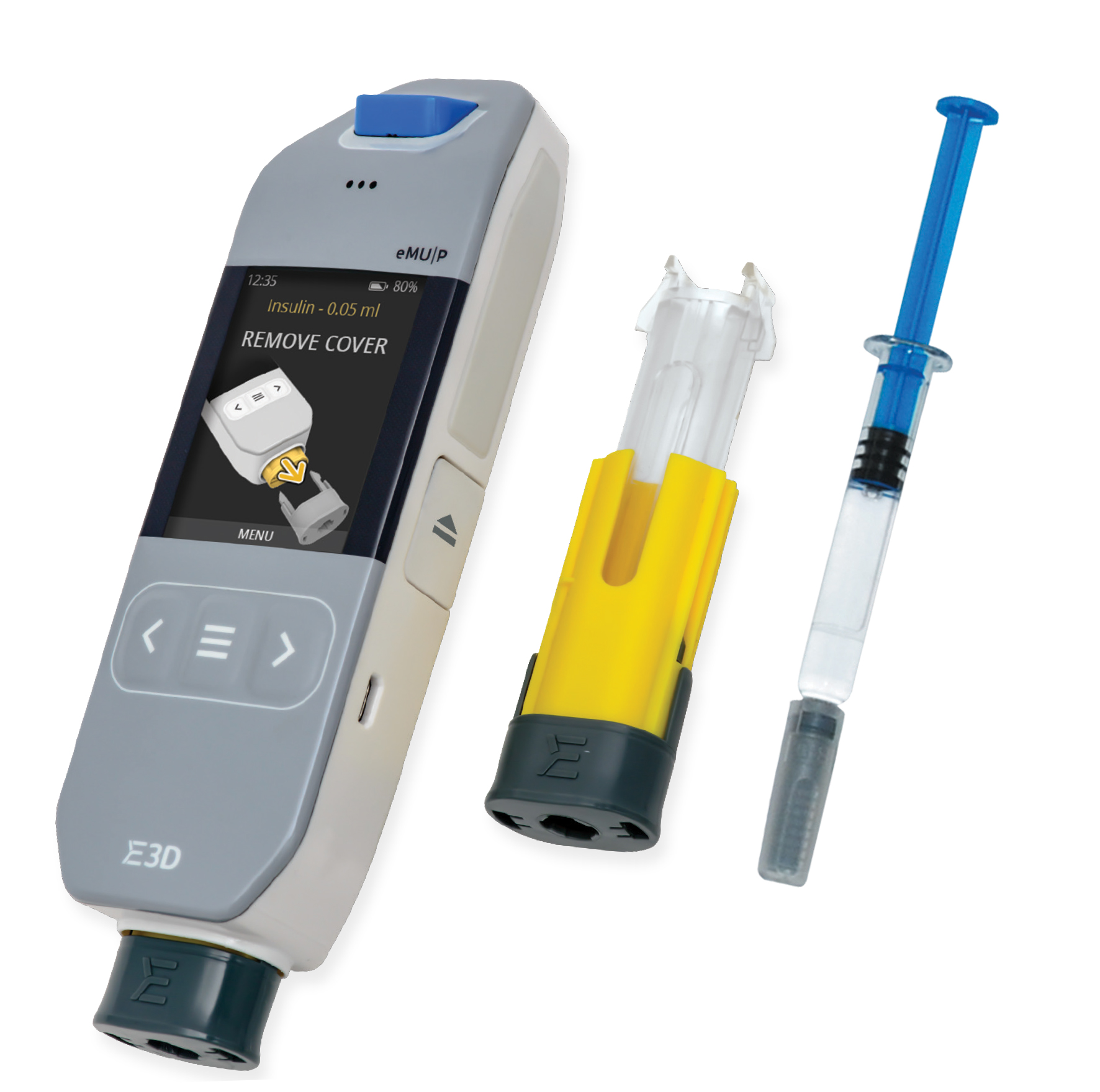
Figure 1: Flexi-Q eMU-P electromechanic re-usable autoinjector and disposable PFS-based cassette.
A variety of factors contribute to non-adherence with a prescribed treatment regimen. Patients sometimes simply forget to take their medication at the right time, while others may not correctly understand the dosing instructions. Patients may fail to complete the full course of a treatment, which can greatly impact its efficacy. While training can go a long way towards helping patients understand how to successfully self-administer and address possible fears and concerns, the problem of forgetting to take medication is less easily addressed and it often falls to family members or other caregivers to ensure medication has been taken on time.
Whilst failure to take medication has obvious implications for a patient’s health, it also impacts on healthcare payers who have a clear interest in finding ways to improve the cost-effectiveness of healthcare. About half of patients suffering chronic illness do not take their medication as prescribed, costing US$100-300 billion (£77-236 billion) annually in avoidable direct healthcare costs in the US alone, according to Springboard.2
CONNECTING DRUG DELIVERY DEVICES
Connected consumer devices have been around for some time, enabling consumers to control everything from their home security system to their garden irrigation system by remote control. While convenient, these systems are often vulnerable to hackers and represent a very real security concern.
As DCA pointed out, when it comes to medical and pharmaceutical devices, connected devices are a very different proposition. To make it to market, new products need to be demonstrably safe and effective – and regulators demand extensive evidence of this. Likewise, it’s also imperative that medical devices are secure, to ensure both safety and confidentiality. However, DCA noted that the benefits of being able to pass information to or from a drug delivery service open up a number of interesting possibilities. Beyond dose reminders, the addition of sensors enables the monitoring of a patient’s condition for side effects or to evaluate the effect of regimen changes.3
PRESERVING PATIENT PRIVACY
It would be understandable for pharmaceutical companies to welcome an opportunity to make use of injection data from a connected device to enhance the design of future products. But while connecting drug delivery devices to the internet represents a technically viable solution, it also opens up valid privacy concerns. Recent scandals in the social media sphere have made consumers very wary of who is collecting their data and what it’s being used for. As healthcare digital strategist Tom Lawrie-Fussey of Cambridge Design Partnership pointed out, context is key when it comes to creating patient-friendly drug delivery devices. It would be a mistake to assume that patients want to have an interaction with your organisation each time they self-administer.4
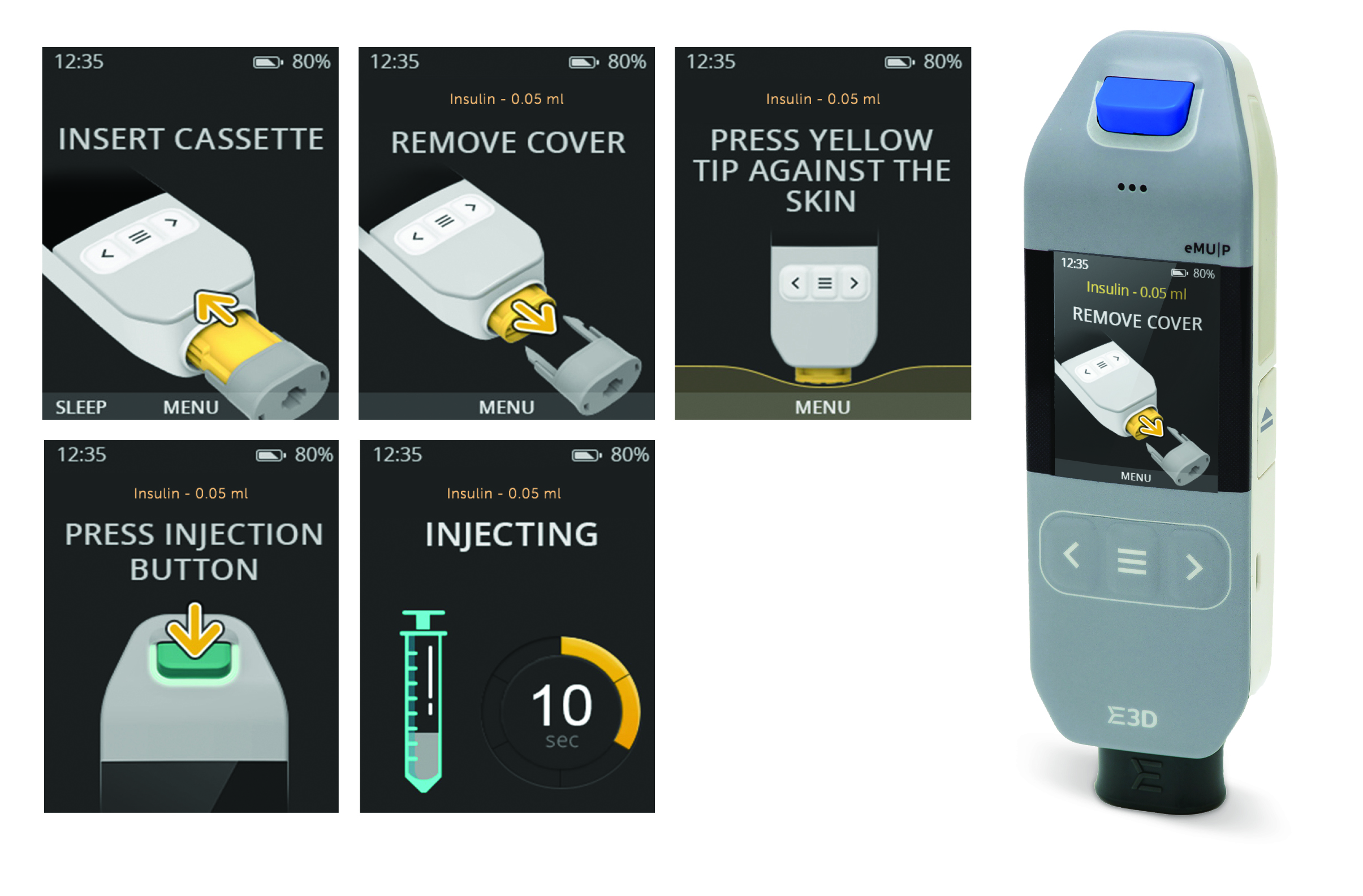
Figure 2: Injection stages and instructions on screen.
This ultimately becomes a consideration of what data is being collected and presented – as well as who is to be granted access to that information. The onus falls on pharmaceutical company suppliers to ensure they have consent before collecting or using patient data. This means asking some key questions, such as: Who will have access to the patient’s data? How will the data be used? How will it be stored? Has the patient given consent for the data to be recorded and shared? These issues must be addressed by pharmaceutical companies to ensure that patient information is only provided to those authorised to receive it and to ensure that patient confidentiality is maintained.
There’s no doubt that connecting devices to smartphones offers a world of possibilities, since all the features of the phone and its related apps can be used – encouraging better adherence, improved patient outcomes and enhanced usability as well as reducing healthcare costs. The benefits are great and growing, as new applications for connected drug delivery devices are brought to market.
STRATEGIES FOR IMPLEMENTING CONNECTIVITY IN DRUG DELIVERY DEVICES
Springboard identified three main strategies for implementing connectivity in a drug delivery device – the first being an add-on, typically to an existing design. The second strategy was upgrading an existing device, which is integrated but does not change the core functionality or use case. The third strategy identified was built-in connectivity, which can change the core functionality and use case. However, Springboard noted that devices with built-in connectivity could be more difficult to make re-usable, which adds inherent cost and environmental impact.2
NEXT-GENERATION ELECTRONIC MULTI-USE AUTOINJECTORS
Various solutions are under development to meet the need for connected drug delivery devices. One such device – the Flexi-Q eMU-P electronic autoinjector designed by E3D – was developed following extensive usability tests involving patients from various groups (gender, age, illness, disability, etc.) All aspects of the Flexi-Q eMU-P autoinjector, including shape, convenience and ease of use, location of buttons, size of display and ideal ratio between injector width and display size were extensively tested and optimised (Figure 1).
The result is a compact, user-friendly and easy-to-use device, designed to solve one of the key reported reasons for non-adherence – failure to understand how the device works. The large-display LCD screen shows each stage of the injection process, providing the patient with clear instructions in real time (Figure 2.)
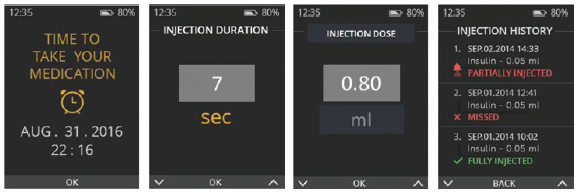
Figure 3: Reminders, data and history display.
Since one of the major problems with adherence is patients simply forgetting to administer the dosage (or failing to remember whether they have done so), the Flexi-Q eMU-P device also provides reminders and injection history (Figure 3). This data greatly assists with compliance, verifying that doses have been administered and giving patients peace of mind.
Specialised mobile apps (Figure 4) enable data regarding injection habits and patient compliance with the prescribed treatment programme to be enhanced. Reminders, logs and injection data can be automatically sent to patients, family members and carers as well as healthcare professionals. Data such as the time, quantity, drug type and whether a full or partial injection was delivered can all be recorded and sent from the Flexi-Q eMU-P via a wireless connection to remote data storage.
Sending data collected from a drug delivery device to a smartphone not only provides patients with a larger screen size on which to view information, if needed – it also offers the ability to provide explicit usage instructions in audio or visual format.
IMPROVED PATIENT SAFETY
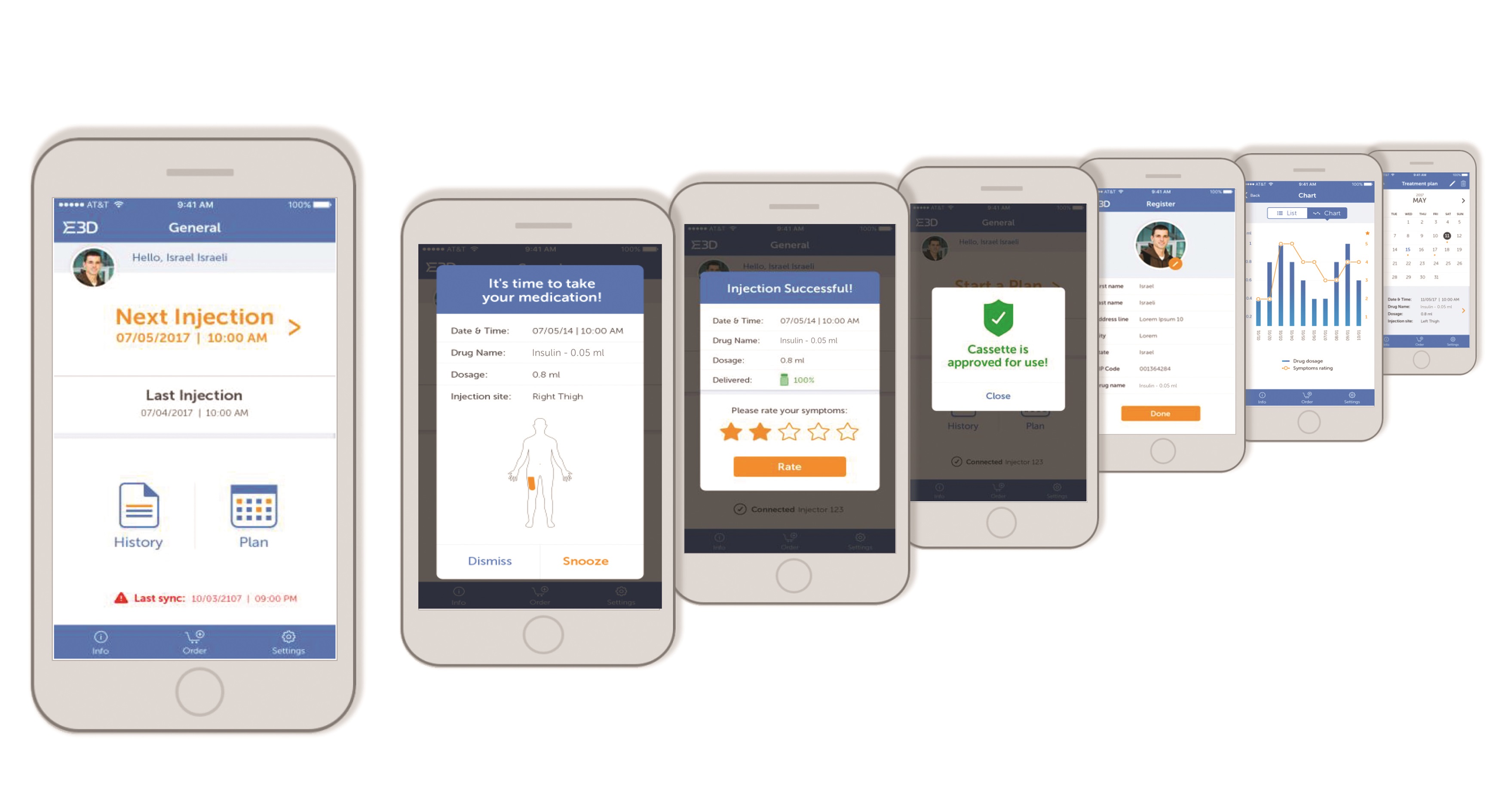
Figure 4: E3D autoinjector app.
Once the patient lifts the Flexi-Q eMU-P autoinjector from the body, the injection process is automatically stopped (even if injection has not been completed) and the cassette in which the drug-filled syringe is contained becomes void. This prevents the patient re-injecting with a previously used syringe and cassette at a later stage. To further ensure patient safety, the disposable cassette includes an RFID component, on which the pharmaceutical company can encode the drug name and dosage, expiry date and anti-fraud barcode, as well as various permissions and definitions. If the drug has expired or is a counterfeit, the Flexi-Q eMU-P will issue a warning and will not allow the injection to proceed.
Additional features include the ability to measure drug temperature before injection is permitted, and the ability for patients to control injection speed in the interests of comfort and reduced injection pain. Drugs with a wide range of viscosities can all be injected using the same device platform.
“The ability to provide home-based self-injection has many benefits.”
PERSONALISING PATIENT CARE
E3D autoinjectors are designed to provide each patient with effective care according to their own specific treatment programme. By making the product safe and easy to use, reducing needle phobia, ensuring delivery of the full dosage and enabling better therapy follow-up, the quality of care, patient safety and adherence to therapy can all be improved. By enabling full supervision of the amounts of drug used, injection logs, reminders to patients, and reports to family members and physicians, every participant in the entire healthcare system can become more involved (even at a distance), thus providing enhanced and effective care. The software enables control of dosage and other therapeutic factors, customising them to individual patient needs after a specific follow-up of injection logs and analysing the therapeutic results attained. This is, in effect, one step closer to so-called precision (or personalised) medicine. Adjustments can be made by patients and/or doctors, depending on parameters predefined by the drug company, allowing high-quality treatment to be administered from a distance.
ADDRESSING ENVIRONMENTAL CONCERNS
E3D’s autoinjectors reduce the storage and waste footprint for both drug manufacturers and patients, thereby reducing any negative environmental impact. As the demand for improved patient compliance and quality of care grows, there’s increasing awareness of production costs. E3D’s re-usable autoinjectors provide an effective solution to the costs associated with self-injection. Disposing of only the single-use cassette element (rather than the entire injector device) after an injection reduces the cost per injection significantly, creating considerable economic advantages.
VERSATILE PRODUCTS TO MEET PHARMA COMPANY NEEDS
The selection of drug delivery devices depends on numerous factors, including formulation, primary package, dosing — and the ability to ensure safe and effective usage. E3D provides re-usable autoinjector units, single-use cassette components, automatic final assembly equipment (for loading drug-filled syringes or cartridges into the cassette) and software for electronic labelling (i.e. RFID chip on cassette) in accordance with each company’s requirements.
The ability to provide home-based self-injection has many benefits: it helps the healthcare industry save costs otherwise associated with clinic-based treatment and offers patients enhanced independence via self-treatment. Appropriate use of smart, connected delivery devices enables remote monitoring to ensure treatments are being administered on time as well as delivering the desired therapeutic results.
Connected drug delivery devices are already here: it remains to be seen to what extent they are embraced by healthcare providers and pharmaceutical companies as well as how they are accepted by the patients they serve.
REFERENCES
- Harvey C, “Normalising Connectivity – Could Unconnected Devices Become the Exception?”. ONdrugDelivery Magazine, Issue 87 (June 2018), pp 56-59.
- Oakley T, “Connected Drug Delivery Sector Overview”. ONdrugDelivery Magazine, Issue 87 (June 2018), pp 4-8.
- Jenkins D, Smith T, “Why we need to think differently about drug delivery device connectivity”. Medtech Media Europe, March 14, 2017.
- Lawrie-Fussey T, “The Smart Approach to Harnessing Data”. ONdrugDelivery Magazine, Issue 87 (June 2018), pp 15-17.

