To Issue 158
Citation: Watts A, Tauber M, “ARCUS®: a Commercialised Technology Enabling High-Dose, Highly Respirable Powders”. ONdrugDelivery, Issue 158 (Apr 2024), pp 48–54.
Alan Watts and Michael Tauber, discuss the requirements and benefits of using spray-dried powders for inhalation, and how ARCUS, used for the successfully commercialised Inbrija® dry powder inhaler, is a prime example of this technology in action.
After more than 20 years of academic research, formulation development, clinical trials and industrial scale-up, Acorda Therapeutics‘ combination product for Parkinson’s patients – Inbrija® – comprised of dry powder particle-engineered levodopa and a breath-actuated device was approved by the US FDA in 2018. This product is one of several commercial orally inhaled products that use spray drying as a means to produce a highly respirable powder capable of efficiently and conveniently delivering a drug payload to the airways (Table 1).
| Product | Spray-Drying Technology | Device | Active(s) |
|
Breztri (AstraZeneca) |
Aerosphere | pMD | Budesonide / Glycopyrronium / Formoterol Fumarate |
|
Bevespi (AstraZeneca) |
Aerosphere | pMD | Glycopyrronium / Formoterol Fumarate |
|
Airsupra (AstraZeneca) |
Aerosphere | pMD | Salbutamol / Budesonide |
|
TOBI Podhaler (Viatris) |
Pulmosphere | DPI | Tobramycin |
|
Inbrija (Acorda Therapeutics) |
ARCUS | DPI | Levodopa |
|
Bronchitol (Chiesi) |
n/a | DPI | Mannitol |
Table 1: Commercial spray-dried products for inhalation.
The spray-dried particle engineering technology used for Inbrija – ARCUS® – enables dosing of 42 mg of API in a single size 00 capsule while minimising excipient content (<8 mg). These engineered powders are consistently taken up into inspired air by a single breath, due to their low density. Unlike traditional lactose-blended dry powder inhaler (DPI) formulations, high respirable fractions of >60% of the nominal dose can be achieved by these spray-dried powders over a broad range of inspiratory flow rates. Clinical data has indicated rapid absorption and improved consistency in plasma concentrations compared with oral formulations. Inbrija uses a device designed for a size 00 capsule, which allows for high powder volume in a single capsule, whereas approximately three size 3 capsules would be required for an equivalent dose from a typical capsule-based DPI.
ARCUS TECHNOLOGY – PARTICLE ENGINEERING BY SPRAY DRYING
The ARCUS technology was initially developed in the labs of Dr Robert Langer at the Massachusetts Institute of Technology (MA, US) and Dr David Edwards at Harvard University (MA, US). This initial development of large porous particles was described in a landmark 1997 Science article titled “Large Porous Particles for Pulmonary Drug Delivery”.1 Shortly after this publication, a start-up company, Advanced Inhalation Research, was launched and began using this approach to develop drug products for systemic drug delivery via the lung. Through various acquisitions and spin-outs, the technology eventually found its home with Acorda Therapeutics in 2014.
“Pulmonary delivery of particles made with ARCUS requires significantly less energy to disperse the powder into an inhalable aerosol, due to decreased interfacial interactions.”
Improved Performance
The drive to develop this technology stemmed from the need to overcome some of the inherent challenges faced when developing dry powders for inhalation delivery. For fine particles (those <5 μm in diameter) intended for lung delivery, cohesiveness is governed by electrostatic and van der Waals forces more so than gravitational or inertial forces. Dispersion of these fine particles can take a lot of energy.
Historically, DPIs have used blends with a carrier excipient, such as lactose monohydrate, to keep these small particles separated, with them adhering to the larger lactose particles rather than each other. Additionally, lactose carriers have served as a bulking agent, allowing for the metering of microgram quantities of API as a blended powder. ARCUS allows for the delivery of drug powder to lungs while minimising and, to some extent, overcoming these inherent challenges (Figure 1).
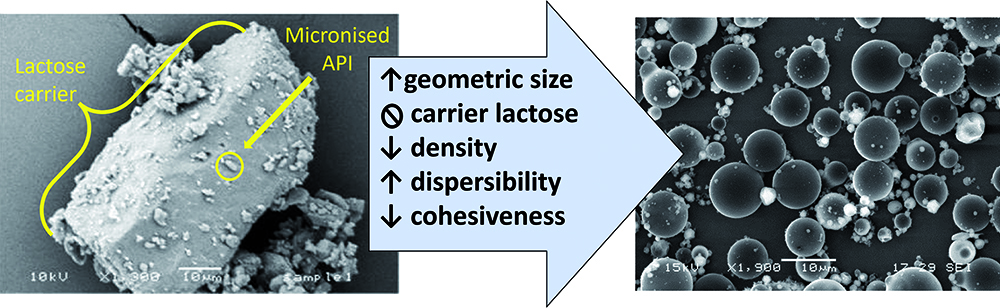
Figure 1: Comparison of a traditional coarse lactose blend with ARCUS technology.
Using a spray drying process, ARCUS generates engineered particles that are easily dispersible, low density and porous, with a mean aerodynamic diameter of ~3 μm. The aerodynamic diameters of these particles are similar to those of conventional aerosol particles, yet their lower density and larger geometric diameter (~10 μm) requires much less energy for powder dispersal, allowing for use by patients with compromised lung function. This improved dispersibility is enabled through a proprietary spray drying process and excipient selection, resulting in low-density particles. Due to the low-density and porous nature of the ARCUS particles, these geometrically large particles exhibit the aerodynamic behaviour of smaller particles.
Pulmonary delivery of particles made with ARCUS requires significantly less energy to disperse the powder into an inhalable aerosol, due to decreased interfacial interactions – specifically, a reduction in particle-particle interactions, including van der Waals forces and electrostatic forces. This reduction in particle-particle interactions reduces agglomeration and leads to improvements in powder flow, dispersion and, ultimately, aerosol generation from a patient-powered DPI device.
From a manufacturing perspective, volumetric metering of small masses is enabled by low bulk density, where a smaller mass will occupy a given metering cavity. However, low-density powders also present filling operation challenges, where special consideration must be given to reduced powder flowability. Complexities and instabilities leading to powder blend heterogeneity are avoided by spray drying composite particles, where each particle contains the same API load. Therefore, bulk powder segregation during manufacture, shipping and storage is of little concern for spray-dried powders.
Solid-State Control
Spray drying approaches often use hydrophobic excipients to improve flow and prevent moisture ingress by preferentially including them on the exterior surface of the particle. ARCUS particles can be made physically stable in an amorphous form with or without a hydrophobic crystalline shell. Within an amorphous powder formulation, excipient selection is dictated by the ability of the particles to maintain a stable glass transition temperature with no evidence of crystallisation or molecular mobility.
As a general rule, a glass transition temperature (Tg) that is approximately 50°C above the formulation’s intended storage conditions is necessary to reduce molecular mobility and minimise the potential for recrystallisation. When matrix material has a lower Tg, closer to the storage temperature, the molecular mobility of the material will increase and molecules will begin to rearrange and self-assemble, which can lead to nucleation, recrystallisation and instability. For Inbrija, a Tg value at or above 75°C is generally observed, making it suitable for storage at room temperature.
Protection from the plasticising effects of residual water, solvent and high relative humidity during manufacture and storage is critical for maintaining the amorphous solid state of these powders. As expected, ARCUS formulations demonstrate an inverse correlation between Tg and residual solvent level (Figure 2). In cases where a Tg is less than 75°C, special considerations for composition, manufacture, packaging and, ultimately, environmental conditions can still facilitate stability.
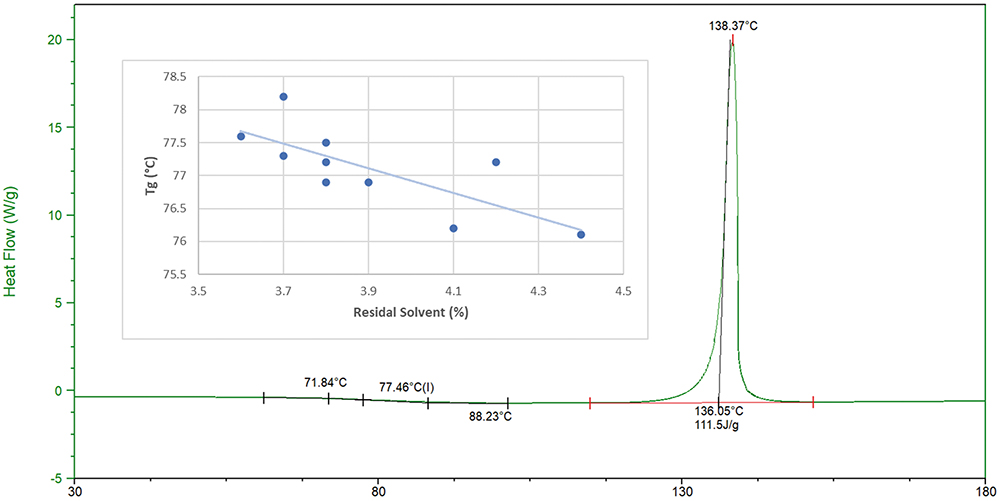
Figure 2: Differential scanning calorimetry thermogram of ARCUS powder and influence of residual solvent on glass transition (Tg).
An amorphous formulation also improves the dissolution rates of poorly water-soluble drugs, which can help improve the rate and extent of absorption. The importance of solubility for pulmonary drugs is reflected in the growing attention to the physiological relevance of dissolution rate-limited APIs that are currently delivered as powders.2 Improvement of solubility through amorphous stabilisation of an inhaled corticosteroid could provide improved efficacy in the lungs and reduce off-target exposure.3
For protein and polypeptide therapeutics, an amorphous state is essential to maintaining conformational and biological stability. The underlying theories supporting stability of a large molecule in an amorphous glass matrix are water replacement and vitrification. Within the particle matrix, excipients that maintain a stable amorphous structure are chosen, which may also be surrounded by a crystalline hydrophobic shell that can combat moisture uptake.
In ARCUS formulations, excipients are selected for their role in:
- Enabling the formation of the particle shell
- Enhancing porosity, dispersibility and aerosolisation
- Maintaining chemical stability.
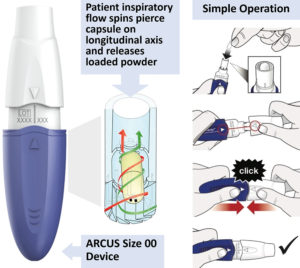
Figure 3: ARCUS device functionality and operation (available for size 00 and 2 capsules).
FDA Approved, High-Capacity Device
One key differentiator between ARCUS and other DPI technologies is the ability to administer high doses. High doses for dry powder inhalation are enabled by a formulation capable of achieving high potency (limited API–excipient ratio) and high repeatability within a single unit dose of powder. Compositions ranging from 10–90% active loading have been developed with ARCUS technology, with typical formulations consisting of ≤ 50% active.
In addition to formulation, the FDA-approved ARCUS device (Figure 3) uses a size 00 capsule, offering over three times the powder capacity of a standard size 3 capsule. Several calculations of total lung dose are given in Table 2 to estimate the quantity of drug load delivered in a single dose of Inbrija, a hypothetical ARCUS powder and a standard coarse blend from the ARCUS size 2 and 00 devices.
| Total Lung Dose (mg) | |||
| API (%) | Size 2 capsule | Size 00 capsule | |
| Inbrija ARCUS powder | 90 | 9.7 | 24.3 |
| Typical ARCUS powder | 50 | 5.4 | 13.5 |
| Lactose blend | 10 | 1.8 | 4.5 |
Table 2: Influences of formulation approach on total lung dose (mg), assuming that half the capsule body is filled with powder (0.45 mL and 0.18 mL). Inbrija is 90% API, 0.1 g/cm3 density, 60% FPF; typical ARCUS powder is 50% API, 0.1 g/cm3 density, 60% FPF; lactose blend is 10% API, 0.5 g/cm3 density, 20% FPF.
“Many spray-dried powders benefit from, or require, specialised facilities capable of maintaining an extremely dry production environment.”
While engineering a spray-dried formulation clearly improves efficiency and enables a higher lung dose, the capacity of the ARCUS 00 device can enable a high lung dose even with a standard lactose blend. It should be noted, however, that 90 mg and 225 mg of bulk blended powder would need to be loaded into size 2 and 00 capsules to achieve these lung levels with the given assumptions. This quantity of powder could require multiple inhalations to clear from a single capsule as the amount of powder that an adult can comfortably inhale in a single breath is approximately 50 mg.4 It should also be noted that these quantities may be poorly tolerated by the patient. If jet milled material were used to produce a high dose product, it might be more effective to use neat powder, avoiding lactose blending completely.5
New Applications
ARCUS has been used to develop a preclinical-stage dry powder synthetic lung surfactant aerosol that has shown potential as a surfactant therapy for pre-term infants supported by non-invasive respiratory support.6 The lung surfactant formulation showed viability for long-term storage as a powder with a stable fine particle fraction (FPF) up to six months at 40°C and one year at 5°C. ARCUS has also demonstrated utility in stabilisation and delivery of nucleic acid payloads as an aerosol without the use of non-viral vector encapsulation, such as in lipid nanoparticles. Future work will focus on the physiological efficacy, uptake and expression of these new modalities.
READY TO SCALE
When evaluating any pharmaceutical technology, it is important to understand its ability to scale to meet clinical and commercial demands, as well as ensuring that a fit-for-purpose manufacturing facility is available. For ARCUS powders, not only is such a facility available, but it has an established track record of regulatory inspection and approval. Catalent Boston acquired Acorda’s 90,000 square-foot manufacturing site in early 2021 and continues to produce and release commercial Inbrija, as well as other clinical-stage spray-dried and encapsulated products.
Spray Drying, Encapsulation and Blistering
Spray-drying capacity within the Catalent Boston site is shown in Figure 4 and includes specialised capabilities for the production of ARCUS powders in addition to other widely used particle engineering approaches. As noted by the PSD-4 and PSD-7 scales, there is ample capacity to produce very large quantities of spray-dried material even in the 1–3% solids loading range, as is typical for inhalation feedstocks. While suitable batch sizes for early clinical trials and orphan-disease commercial products can be obtained using a pilot scale PSD-1, evaporation capacity peaks at approximately 5 kg/hr and limitations of multi-day process stability and resourcing must be considered. Batch sizes in the 10s or 100s of kilos will find a better fit at the PSD-4 and PSD-7 scales, respectively.
| PSD-1 | PSD-4 | PSD-7 | |
| Batch (kg) | 0.1–3 | 10–90 | 30–200 |
| Drying gas (kg/hr) | 90–175 | 1000–1750 | 4000–11000 |
| Liquid feed (kg/hr) | 1–5 | 30–100 | 100–300 |
| Feedstock volume (L) | 5–150 | 750–4500 | 1400–10000 |
Figure 4: Spray dryer scale and capacity at Catalent Boston.
For ARCUS and other spray-dried powders for inhalation, encapsulation is often used to contain powders in pre-metered unit doses that are compatible with a variety of multi-use DPI devices, including the ARCUS size 00 and 2 devices. While spray drying yields highly respirable powders with low to moderate levels of excipients required, these powders often have poor flow properties. With no large, coarse particles to improve flow and the need for low fill weights, the filling operation can often be challenging with traditional encapsulators.
At the Catalent Boston site, drum filling technology with specialised powder feeding systems has been designed to accommodate high Carr’s index powders at fill rates of up to 100,000 capsules per hour. At mid-speed, this could encapsulate a 5 kg batch of 20 mg filled capsules in five hours. As many inhalation APIs are categorised as potent, enclosures to protect from dust generated by the filling operation are also in place for this unit operation.
Special consideration must be given to the handling and exposure of bulk and even encapsulated powders at this step in the manufacturing process. Because unprotected filled capsules are not fully protected from the environment, rapid blistering in a single controlled manufacturing setting is also desirable, and is completed at the site prior to sleeving for shipping to a secondary pack assembler.
Need for Moisture Protection, Low Relative Humidity
Many spray-dried powders benefit from, or require, specialised facilities capable of maintaining an extremely dry production environment. Moisture exposure during production, even momentarily, can render moot the careful controls placed over bulk spray-dried powder and capsule dryness. Catalent Boston maintains a relative humidity (RH) less than 20% throughout encapsulation and blistering operations to ensure that sensitive powders are not exposed to destabilising moisture. Lower controls (<10% RH) have also been demonstrated in designated suites for operations with extreme moisture sensitivity.
CONCLUSION
Spray drying to manufacture powders for inhalation is now a well-established practice in the pharmaceutical industry that allows for the production of highly respirable, high-dose formulations. ARCUS technology incorporates the benefits of spray drying with a capsule-based high-capacity device. As one of only three commercially approved technologies for spray drying inhalable powder, ARCUS provides an opportunity to design an inhalation product with an established and successful regulatory track record.
With development, commercial-scale manufacturing and analytical equipment already in place at a single site, the supply chain is simplified, and tech transfers are largely eliminated. With the approval of Inbrija in 2018, the 20-year-long path from invention to market was completed, creating a de-risked and, likely, more timely opportunity for follow-on products.
To learn more about Catalent’s inhalation offering, visit: www.catalent.com/specialty/inhalation.
REFERENCES
- Edwards DA et al, “Large porous particles for pulmonary drug delivery”. Science, 1997, Vol 276(5320), pp 1868–1871.
- Hastedt JE et al, “iBCS: 1. Principles and Framework of an Inhalation-Based Biopharmaceutics Classification System”. Mol Pharm, 2022, Vol 19(7), pp 2032–2039.
- AboulFotouh K et al, “Amorphous solid dispersion dry powder for pulmonary drug delivery: Advantages and challenges”. Int J Pharm, 2020, Vol 587, Article 119711.
- Son Y-J, Miller DP, Weers JG, “Optimizing Spray-Dried Porous Particles for High Dose Delivery with a Portable Dry Powder Inhaler”. Pharmaceutics, 2021, Vol 13(9), Article 1528.
- Brunaugh AD et al, “Excipient-Free Pulmonary Delivery and Macrophage Targeting of Clofazimine via Air Jet Micronization”. Mol Pharm, 2017, Vol 16(11), pp 4019–4031.
- Walther FJ et al, “Efficacy, dose-response, and aerosol delivery of dry powder synthetic lung surfactant treatment in surfactant-deficient rabbits and premature lambs”. Respir Res, 2022, Vol 23(1), Article 78.

