To Issue 139
Citation: Willoughby A, “Assessing the Environmental Impact of Global Supply Chain Logistics and Supplier Selection”. ONdrugDelivery, Issue 139 (Oct/Nov 2022), pp 30–34.
Alastair Willoughby looks at the factors affecting sustainability when considering global supply chain logistics and supplier selection.
Increasingly, medical device developers are considering ways to build sustainability into their device designs, from low-impact polymers to reducing device complexity. While this has an important role to play in reducing overall emissions, in reality, the design of the device often makes up only a small part of its overall environmental impact. Transportation, especially carbon-costly methods such as air freight, often contributes a much larger carbon output than the device manufacture itself. If companies are serious about meeting their carbon targets, it is essential to consider factors such as global supply chain logistics and supplier selection throughout the device development.
“Selecting a manufacturing plant in a country heavily reliant on coal-fuelled power will create more carbon output than one in a country using more wind and solar energy.”
Building an evidence-based understanding of the environmental impacts of different supplier choices, transportation routes and design decisions is no easy task. There are many factors and complexities to take into account, from manufacturing methods to energy sources to power them. Luckily, tools such as lifecycle analysis (LCA) can help decision makers to navigate these complexities, enabling insight-driven decisions to be made throughout a development.
WHAT IS LCA?
LCA is a methodology for assessing the environmental impact of a product or process over its lifetime. It can be used to assess the carbon footprint of multiple aspects of a development, from individual parts to transport options and manufacturing processes. The stages and approaches of an LCA are set out in ISO:14040, covering four main stages (Figure 1):
- Goal and scope definition
- Inventory analysis
- Impact assessment
- Interpretation.
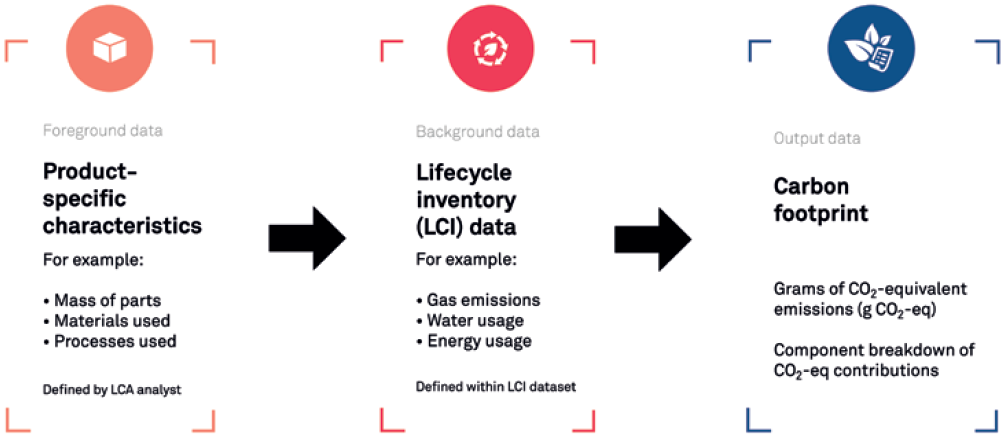
Figure 1: LCA process for medical device development.
LCAs are used across multiple industries, meaning there is often public data available for certain processes that can be fed in. In the medical industry, however, there is typically less data available for medical-specific processes, such as how much energy is required to run a clean room for use during an assembly process. Research, coupled with a strong understanding of these industry-specific processes, is often needed to build a more complete representation of the data needed for a medical device LCA. It is also important to note here that, while an analytical tool, there will always be some scope for interpretation in the results. However, it is still a valuable tool for helping to shape our thoughts and decisions.
LCA SCOPE DEFINITION
The scope of an LCA can vary, depending on your goals and the data available. As illustrated in Figure 2, a cradle-to-gate LCA considers the process from raw material extraction to the point the product is ready for transport distribution, i.e. the factory “gate”. A cradle-to-grave LCA considers a much wider scope, including the transportation, distribution and use of the product, as well as disposal and waste. A cradle-to-cradle LCA can also be conducted, which considers reuse and recycling as well.
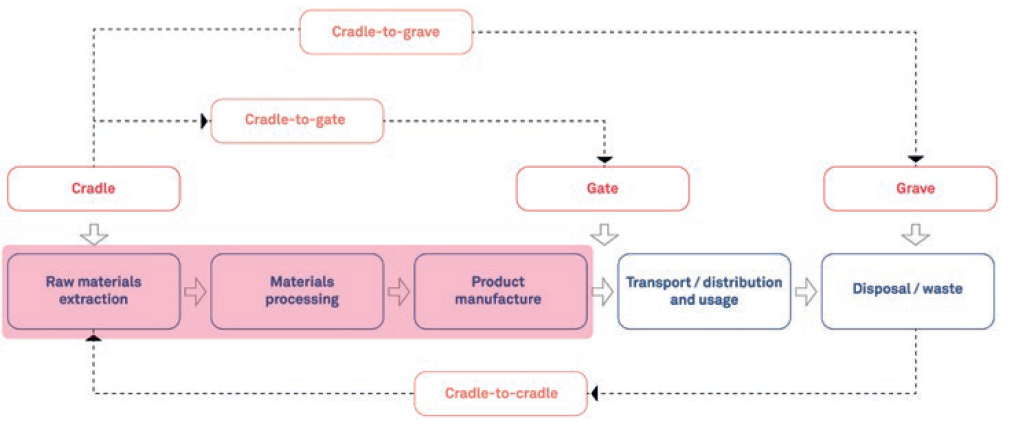
Figure 2: Example of LCA scopes.
The scope you choose for your development will often depend on what you are trying to achieve. You may be trying to simply benchmark your device, for example, or perhaps you are interested only in what you have direct control over in the development process. How much data is available, as well as its reliability, can also impact the scope of your LCA.
Within your scope definition, what you choose to include and not include can make a lot of difference to the overall picture your LCA provides. For example, a simplified cradle-to-gate LCA assumes that transport only takes place after the manufacturing process. A more comprehensive representation of this process would also take into account the transport of materials and components within the manufacturing stages too, for example.
“Device developers should also consider decisions that could help improve other carbon-costly factors, such as transit packaging.”
WHAT SHOULD AN LCA COVER?
As mentioned, an LCA can have a broad or relatively narrow scope. The following aspects are some of the most important to consider when determining the environmental impact of a device.
Device Components
A basic LCA for a medical device might only cover the components of a device and the carbon footprint they create. This can be useful for understanding the impact of different parts and features of the device, such as connected add-ons. It can also help to understand the impact of different manufacturers and suppliers. For example, selecting a manufacturing plant in a country heavily reliant on coal-fuelled power will create more carbon output than one in a country using more wind and solar energy.
While these insights can be used to inform design decisions, the device components are only part of the wider picture. To fully understand the environmental impact of your development, you also need to consider transport and packaging.
Transport
The location of the organisations involved, materials that need to be shipped between organisations, and the route and method of transport all play a significant role in a product’s overall carbon footprint. For example, a sub-assembly in the Far East could be sea or air freighted into Europe where it is then assembled into the device. This additional travel can lead to a major increase in carbon footprint, especially when using carbon-costly methods, such as air freight. Factors such as temperature control during shipping and the volume and duration of the material also need to be considered.
Transit Packaging
When shipping devices or components over any distance, transit packaging will be required. For example, syringes will typically be placed in single-use trays and tubs, which will then be placed into bags, boxes and pallets. Sub-assemblies for autoinjectors often need to be transported before being put together into the final device, each of which require packaging. Once completed, the device will then also be placed into its final consumer-facing packaging. Often, transit packaging is not reused, meaning the manufacturing carbon costs of this packaging, alongside the weight it adds to shipping and subsequent carbon footprint, also need to be considered as part of the total impact.
A COMPARISON OF DIFFERENT LCA SCOPES
To illustrate the difference between different LCA scopes, three LCAs were conducted based on the same fully functional autoinjector with needle safety system. The device is made up of 12 components.
Simple LCA
The graph in Figure 3 shows the results of a simple LCA, which focuses on the manufacture of the device components and primary pack. The resulting carbon output is 141 g CO2 equivalent (eq). An assessment of this level might be suitable if you are a device developer and primarily interested in the sustainability factors you have most control over – such as the impact of adding or removing features or components or making changes to the material used. However, as noted before, this makes up only part of the wider picture.
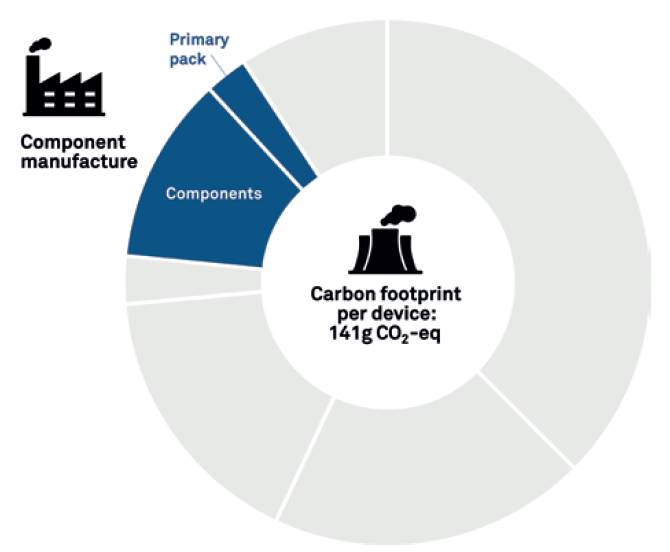
Figure 3: Basic LCA.
Complex LCA
In Figure 4, we can see a more complex LCA that goes a step further to not only consider the device components but also the energy associated with assembling the device, syringe filling and final packaging. In this assessment, the assembly and product packaging increase the carbon output by around 86%, from 141 to 263 g CO2 eq.
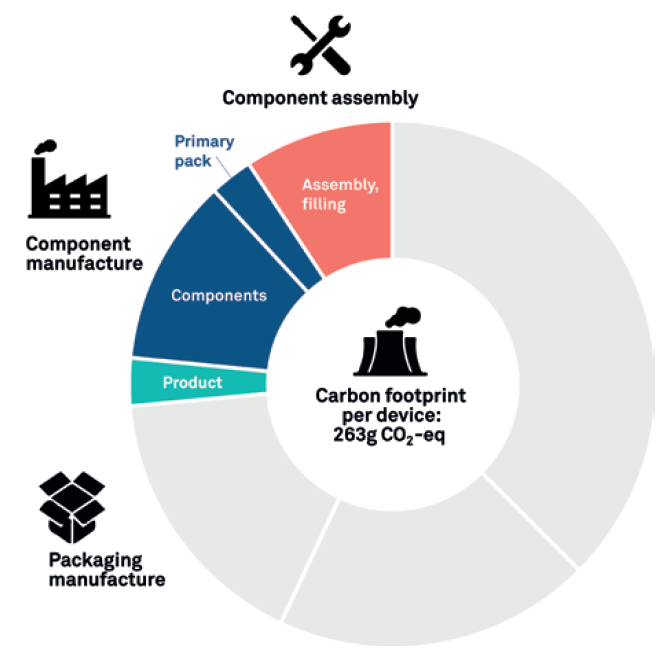
Figure 4: Developed LCA.
A More Comprehensive LCA
Figure 5 shows the results of an LCA that includes shipping and transport during the various stages of device development. While shipping routes and methods vary with each device, it has been assumed that some components and assemblies are made in the Far East and then air freighted to Europe. Other components have been analysed as being made in Europe and transported on the ground via lorries. The carbon footprint of manufacturing the drug has not been included in this assessment. However, the mass of the drug has been factored in as it is transported for assembly. The assessment also does not include shipment to a distribution centre or to consumers, covering transport only to the point it is manufactured and ready to distribute – the final gate. In this example, if the product was assembled in Europe but needed to be distributed in the US, this could add a significant carbon footprint to the overall results.
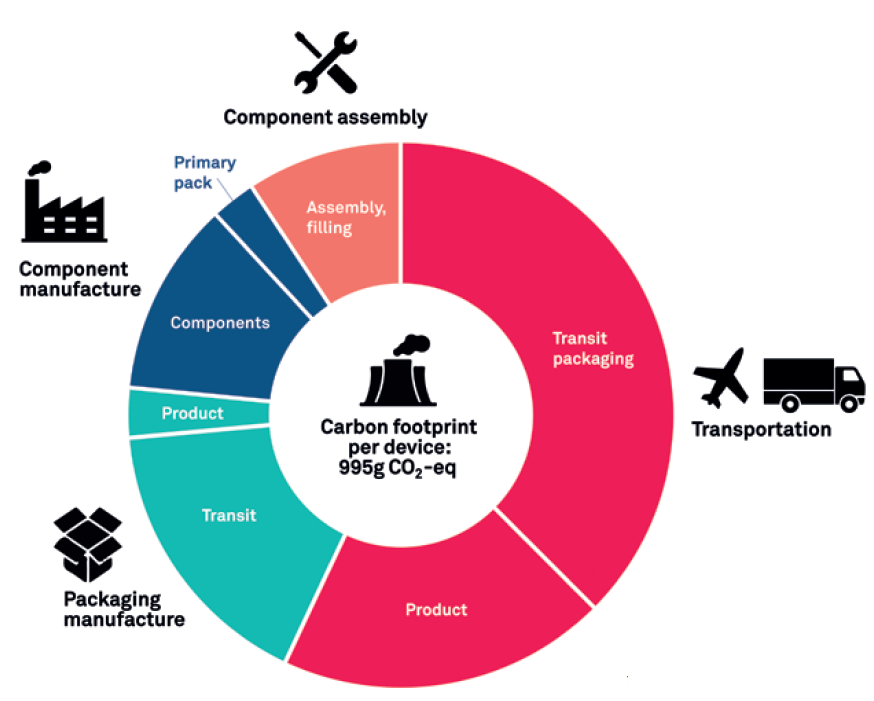
Figure 5: More comprehensive LCA.
“Device developers should be actively questioning suppliers about their sustainable practices and credentials, as well as their plans and targets for the future.”
Within the scope of this LCA, transport makes up almost two-thirds of the product’s overall carbon footprint, increasing the total to 995 g CO2 eq. While air freight makes up a disproportionately large part of this, it is notable that around two-thirds of the transport carbon footprint was produced from transit packaging alone. Clearly, factors such as these should be a priority for device manufacturers to consider.
HOW CAN WE INFLUENCE OUR CARBON FOOTPRINT?
Design Decisions
Some of the common approaches currently being considered around sustainable medical device development focus on the device itself, aiming to minimise or improve the sustainability of the materials used, simplify the device where possible and design for end of life. While these are important steps, device developers should also consider decisions that could help improve other carbon-costly factors, such as transit packaging. For example, if two sub-assemblies are required, they might be nested together to reduce the volume of packaging.
Packaging
In addition to minimising packaging through design choices, more sustainable packaging choices can also be implemented. We are already seeing improvements made in this space, such as reducing the material thickness in thermoformed trays. At scale, this could greatly reduce the manufacturing requirements, material mass and carbon impact of transit packaging.
There is also the potential to reuse packaging. While there are, of course, challenges around this, such as quality management and maintaining cleanliness, integrity and traceability, packaging is still one of the easiest elements within the product lifecycle to reuse without significant energy input.
Procurement Decisions
Another factor that can influence environmental impact, particularly for pharmaceutical companies, is who you are buying from and how you are moving products. Where your supplier is based and how they plan to transport products to your distribution or assembly sites should be an important question when making your procurement decisions. Of course, you may be limited in supplier choice for certain technologies but, where there is a choice, factors such as location, energy efficiency and eco credentials should be considered. Device developers should be actively questioning suppliers about their sustainable practices and credentials, as well as their plans and targets for the future. Procurement groups should also consider the impact of different shipping routes as part of contract negotiations and shipping plans.
SUMMARY
Identifying the carbon footprint of your device development is a complex task. However, it is an important one if we are to truly make changes that will reduce our environmental impact. How you define the scope of your LCA plays a major role in identifying where changes could really add value. As device developers, it is important to consider not just the environmental impacts of manufacturing a device itself but also wider impact factors, such as transit packaging and transport, which often contribute the most carbon footprint.
By considering the supply chain from the start of the development and procurement process, as well as making more sustainable design choices, we can begin to enact real positive change.

