Citation: Guillet A, Deng E, Sysko R, Armani V, Salido MJ, Barbé F, Holloway E, “Biocorp’s Mallya – Chronicling a Success Story”. ONdrugDelivery, Issue 121 (Jun 2021), pp 6–10.
Arnaud Guillet and colleagues from across the industry, discuss Bicorp’s Mallya add-on device for disposable insulin pens, providing details on the main country launches and key software integrations, as well as presenting initial feedback from healthcare professionals and patients.
Diabetes is the most common chronic disease in the world, affecting over 460 million people. If left untreated, diabetes can lead to serious complications, which is why self-monitoring and regular disease management are so important.
In recent years, growing interest and research in the field of connected health have led to major advances in diabetes management. Innovations have taken place in the field of glucose monitoring, with the first connected blood glucose monitors (BGMs) and continuous glucose monitors (CGMs) reaching the market. At the same time, dozens of diabetes management software applications have appeared on the market, aiming to ease the daily life of patients.
There has also been innovation in the field of treatment management. The combination of CGMs, AI and connected insulin pumps has led to fully automated diabetes management systems. However, these “closed-loop systems” are only available to a limited number of users due to their high cost and many patients being reluctant to use such invasive solutions. Pen injectors are likely to remain the primary mode of insulin delivery for the foreseeable future, for most Type 1 (T1) diabetes and a huge majority of Type 2 (T2) diabetes.
With this in mind, major players in the diabetes space are looking into smart pen options to close the loop and complete their service offering. For instance, Medtronic (Dublin, Ireland), a leader in the insulin pump market, recently acquired Companion Medical (San Diego, CA, US) and its InPen™, a Bluetooth Low Energy (BLE) enabled reusable insulin injector, to expand their addressable market. Other smart reusable pen options, such as NovoNordisk’s (Bagsværd, Denmark) NovoPen 6® and NovoPen Echo® Plus, have been launched this year in Europe.
However, most people with diabetes use disposable insulin pens rather than reusable ones. This is due to a variety of factors, including convenience, physician/patient awareness and preference. With the goal of simplifying life and improving the well-being of people with diabetes using disposable insulin pens, Biocorp saw an opportunity to answer an unmet need by developing and marketing Mallya (Figure 1).

Figure 1: Mallya is a connected add-on device for disposable pen injectors.
Since Mallya has now reached the market and become a concrete experience for thousands of patients the world over, the authors would like to present the main initiatives engaged in more detail, and provide some initial feedback from end users. This review will begin with a specific focus on two of the main country launches in France and Taiwan through Biocorp’s key partners, Roche Diabetes Care and Sanofi, then moving on to integration with software as a medical devices (SaMD) players that are adding valuable features to Mallya, such as basal insulin titration or a bolus calculator, and finally highlighting Mallya’s benefits to end users through a patient testimonial and the feedback from a clinical study using Mallya devices.Mallya automatically captures, reports and displays the insulin injection data, including the number of insulin units (IU) delivered, date and time, in a mobile application acting as a digital logbook. The device is accurate and compatible with major disposable insulin pens – Solostarfrom Sanofi, KiwkPen from Eli Lilly and FlexPen from Novo Nordisk – and began commercialisation in November 2020. After a major partnership was signed with Sanofi for the global distribution of Mallya, Biocorp unveiled an additional distribution agreement in 2020 with Roche Diabetes Care for the French market. Mallya is already available in France, Romania, South Africa and Taiwan, and additional launches are planned for 2021, including Europe, Asia, the Middle East, Latin America and ultimately the United States.

Figure 2: Biocorp’s Mallya links with Roche Diabetes Care’s Gluci-Chek app.
MALLYA LAUNCH IN FRANCE WITH ROCHE DIABETES CARE
With Valérie Armani, Head of Marketing, Healthcare Development and Innovation at Roche Diabetes Care, France.
For more than 40 years, Roche Diabetes Care has been developing solutions to meet both the needs of patients with diabetes and healthcare professionals, with the goal of helping patients think less about the daily management of their disease. To this end, Roche Diabetes Care France has created an open ecosystem connecting devices, such as the company’s Accu-Chek® glucometers, to digital solutions for patients and healthcare professionals. With the connection of Mallya to the Gluci-Chek companion app (Figure 2), patients have one more option to lighten the cognitive burden of their disease. Downloaded by more than 60,000 people, Gluci-Chek combines three major functionalities:
- A carbohydrate calculation tool
- A blood glucose self-monitoring diary
- A graphic visualisation of glycaemic results.
Once Mallya and Gluci-Chek are connected, patients no longer need to report their insulin doses manually; their blood glucose logbook collects and displays accurate data for them to rely on. Furthermore, when shared with them through the dedicated Roche Diabetes Care Platform, insulin data allow healthcare professionals to monitor the progress of their patients’ disease, refine their interpretation and help adjust treatment decisions.
MALLYA LAUNCH IN TAIWAN WITH SANOFI AND HEALTH2SYNC
“Patients using the connected ecosystem can seamlessly track, synchronise and plan reminders for their insulin injections thanks to Mallya and the Health2Sync platform, which empowers them to manage their diabetes.”
With Francois Barbé, Head of Integrated Care for the diabetes franchise at Sanofi, and Ed Deng, Co-Founder and CEO of Health2Sync.
The prevalence of diabetes in Taiwan has grown in line with the global trend; the most recently published data states that, in 2014, around 2.2 million people in a population of 23.4 million live with diabetes mellitus. While several treatment options have entered the Taiwanese market in the last decade, there is still a significant unmet need, with only 42% of these people having well-controlled blood sugar levels. Additionally, survey data published in 2019 states that, in 2014, the rate of insulin use in Taiwan was low (13%), with people with T2 diabetes starting injectable therapies on average between 10 and 12 years after their initial diagnosis.
Since 2007, the Taiwanese diabetes healthcare system has evolved towards promoting higher quality care. Healthcare organisations are now increasingly leveraging digital solutions to improve the quality of diabetes care, and the healthcare technology ecosystem is growing to further support both healthcare professionals and patients.
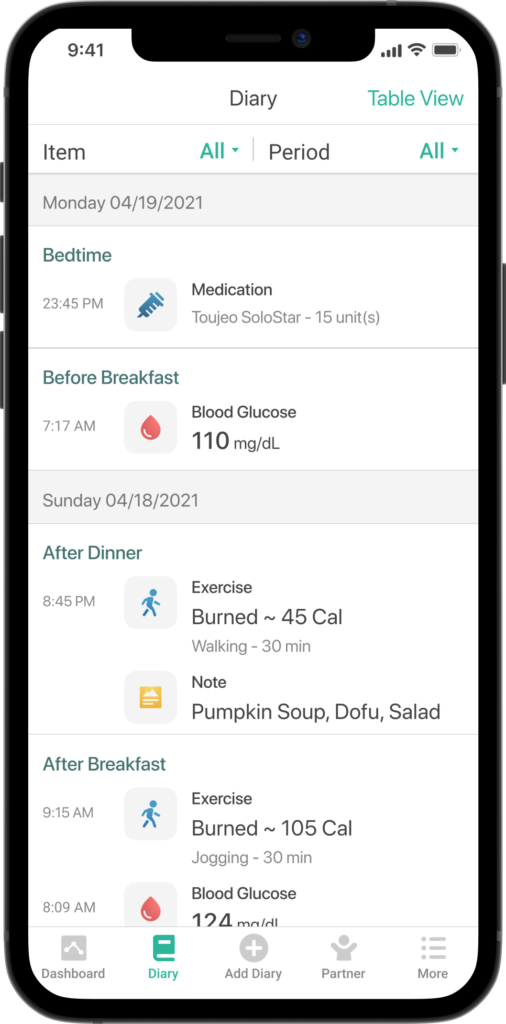
Figure 3: Combined, Sanofi’s distribution network, Health2Sync’s digital platform and Bicorp’s Mallya device are able to provide a digital logbook for diabetes care.
In this context, Sanofi, Health2Sync and Biocorp are realising their shared ambition to improve chronic disease management through a fully connected ecosystem for diabetes management in Taiwan. This connected ecosystem comprises Sanofi’s insulin-based treatment options and strong distribution network, Health2Sync’s digital healthcare platform that enables connectivity with various sources of patient data, and Biocorp’s Mallya connected insulin pen cap, which is provided to patients to track their insulin injections (Figure 3).
Patients using the connected ecosystem can seamlessly track, synchronise and plan reminders for their insulin injections thanks to Mallya and the Health2Sync platform, which empowers them to manage their diabetes. Healthcare providers are given access to real-time insulin injection data alongside other patient-tracked behavioural data through the patient management platform, providing a new layer of information on adherence to insulin treatment and helping to optimise the clinical decision-making process.
Prior to launch of the connected ecosystem for diabetes in Taiwan, Sanofi and Health2Sync worked hand-in-hand to accelerate digital adoption and transformation in clinics and hospitals. Since late 2020, more than 150 Taiwanese clinics have joined the programme, with over 20,000 patients recruited on the Health2Sync platform. Sanofi initiated distribution of Mallya across Taiwanese clinics and hospitals in May 2021.
Other integrations or technology partnerships signed with SocialDiabetes, Amalgam Rx and Diabeloop have opened a new high potential international market and enabled rapid adoption of Mallya.
INTEGRATION WITH AMALGAM RX’S ISAGE PLATFORM
With Ryan Sysko, CEO of Amalgam Rx.
Amalgam Rx is a leader in bringing providers, life sciences and digital solutions together. For more than 15 years, the company’s team has been reimagining care delivery and creating lasting change across the chronic care ecosystem. Working in partnership with many of the world’s leading life sciences companies and healthcare systems, Amalgam Rx has built an innovative platform for rapidly developing and scaling digital solutions. iSage, Amalgam Rx’s flagship product, was the first US FDA-approved insulin titration application that supports all basal insulins. iSage will soon have additional regulatory clearance and be deployed on four continents. Amalgam Rx shared findings from its first clinical trial of iSage during the poster presentations at the American Diabetes Association’s (Arlington, VA, US) 79th Scientific Sessions. The results of the study showed that patients can benefit from the use of automated insulin optimisation support. The outcomes included the following:
- On average, patients in the treatment arm reduced their A1C by 1.04% (p=0.002) from baseline by study termination.
- Patients with a beginning A1C of greater than 8% reduced their A1C by 1.88% (p=0.005) from baseline by study termination.
- The average starting and app-directed final doses of insulin increased by 39% (p=0.047), with less than 2% of the fasting glucose readings recorded in iSage below 70 mg/dL.
Pairing iSage and Mallya simplifies insulin dose capture for patients with diabetes. The combined solution creates an easy and accurate way for patients to record their basal insulin intake while receiving automated guidance on how to adjust their dose over time. This simplifies the often complex and confusing journey for T2 diabetics starting on basal insulin and, to date, offers the most powerful environment for diabetes digitalisation and patient support.
INTEGRATION WITH SOCIALDIABETES
With María Jesús Salido, Co-Founder and CEO of SocialDiabetes.
“The association between the SocialDiabetes bolus advisor, which provides insulin recommendations to patients before each meal, and Mallya is particularly powerful for patients whose therapy requires multiple daily insulin injections.”
SocialDiabetes is a complete digital health platform with a mobile app and a medical dashboard that connects physicians with patients, in real time, to optimise outcomes through smart connectivity and data insight. In detail, the platform developed by SocialDiabetes (CE marked and FDA 510(k) cleared) allows self-monitoring of insulin doses and offers personalised recommendations to patients. The collected data feeds a telemedicine platform using augmented intelligence for physicians and specialised call centres.
Patients using the combined solution are able to automatically synchronise the data recorded by Mallya, thus benefiting from the complete monitoring offered by combining glycaemic data analysis and insulin dose feedback. The association between the SocialDiabetes bolus advisor, which provides insulin recommendations to patients before each meal, and Mallya is particularly powerful for patients whose therapy requires multiple daily insulin injections. The information collected is transmitted to healthcare providers to give them a complete overview of their patients’ status and compliance with their treatment plans. This improves the quality of dialogue between the healthcare provider and their patients, enabling adjustments of treatment plans and personalised recommendations.
SocialDiabetes is a leader in digital management of diabetes in Spanish-speaking markets. The company, headquartered in Barcelona and with offices in London and Ciudad de Mexico, serves 300,000 patients and more than 20,000 physicians. The company’s alliance with Biocorp represents a new integrated care ecosystem for delivering an effective, consistent and meaningful response to the global diabetes challenge.
END USERS AS THE ULTIMATE JUDGES
All these efforts to build the relevant service offering and ecosystems are in pursuit of the same goal: delivering a solution that simplifies diabetes management for patients and brings greater comfort and serenity, and helps doctors better monitor their patients and adjust their recommendations based on objective data. Therefore, it is important to conclude this article by presenting some feedback from healthcare professionals and patients (Box 1) who have used Mallya for the past few months.
BOX 1: MALLYA USER TESTIMONIAL – “MALLYA ALLOWS ME TO BE SERENE”
“I have been a Type 1 diabetes patient for several years now. I was contacted by Biocorp last year to preview Mallya. The simple and elegant design immediately appealed to me. I appreciated the technology added to my insulin pen. Keeping a track of every injection is a real plus. It offers me a lot more peace of mind concerning my injections and allows me to keep better track of my insulin doses, especially when I am not thinking straight.
“On top of that, this device is easy to use, useful and very fun. The application to which Mallya is linked is clear and intuitive. You do not need to be computer literate to use it. From the first time I used it, I was completely convinced by Mallya and, today, I trust it to monitor my treatment. This smart device allows me to be serene and to better live with my diabetes.”
Clinical Study Using Mallya at Croydon University Hospital
With Edward Holloway, MD, Clinical Lead for the paediatric diabetes service at Croydon University Hospital, UK.
The paediatric diabetes services at Croydon University Hospital, UK, has a cohort of 150 patients. The demographics include a high percentage of non-white ethnic groups (55%) and patients from the lowest quintile of the index of social deprivation (23%). The percentage of patients with very high A1C (>80 mmol/mol) is also very high (24% in 2020).
This group of patients are at the highest risk of experiencing serious complications from their diabetes and requiring emergency hospital admission. Therefore, they need greatest attention from clinicians working in the field. Over recent years, technological improvements in diabetes care, such as pump therapy and CGMs tend to see greatest uptake by those with the best diabetes control and are shunned by the cohort of patients with very high A1C who stand to gain the most.
The reasons for persistent high A1C are complex and multifactorial. However, missed doses, under dosing and overdosing with insulin are much more common in this demographic, and it can be very hard for clinical teams to have a productive discussion about these issues with patients, as monitoring focuses on glucose control with very little in the way of options for data collection on insulin administration with non-pump users.
The hospital ran a pilot study using Mallya devices from December 2019, however it was cut short by the covid-19 pandemic. Few patients with very poor diabetes control (including those with A1C levels as high as 130 mmol/mol) confirmed an important role for Mallya devices in informing the clinical team about the true insulin usage and allowing for a more open discussion to explore the themes behind missed/inappropriate dosing of insulin. As such, Mallya offers an exciting insight into the real-world use of insulin in adolescents with very high A1C, and has been incorporated into the hospital’s “high A1C pathway” as a novel tool to help guide these patients towards improved diabetes care.
CONCLUSION
This large-scale launch is an exciting moment for Biocorp. Initial feedback from the daily use of Mallya by patients all over the world is very promising and strongly reinforces the company’s confidence in the usefulness and reliability of the platform. In parallel to this intense activity in the field of insulin, Mallya has already expanded into other therapeutic areas involving drug delivery through pen injectors, such as growth hormone deficiency and fertility. Although form factor, device requirements and treatment objectives slightly vary from one area to another, the core promise of the technology remains intact; to record the dialled dose with the highest level of accuracy, while minimising the usability impact for patients and implementation constraints for the healthcare industry.
Beyond simply monitoring injection, Mallya presents a means to offer reassurance and peace of mind to patients, providing them with evidence that they have properly injected their medication and giving them confidence that they are on the right track to achieve their treatment objectives, whether it’s improving their quality of life, reaching optimal growth or becoming a parent. Aiming to further improve patients’ compliance and follow up, Biocorp continues to work on its medical device platforms, confirming its ambition to be a world leader in the market.
BIBLIOGRAPHY
- Staines R, “Health2Sync and Sanofi to digitally manage diabetes in Taiwan”. Pharmaphorum News, September 17, 2020.
- “Biocorp and Sanofi Announce a Partnership to Fit SoloStar Insulin Pens With Mallya Technology Worldwide”. Press Release, Biocorp, December 12, 2019.
- “Biocorp signs two new partnerships with Mallya technology”. Press Release, Biocorp, February 17, 2021.
- Sheen JY et al, “Trends in prevalence and incidence of diabetes mellitus from 2005 ro 2014 in Taiwan”. J Formosan Med Assoc, 2019, Vol 118(2), pp S66–S73. (DOI: 10.1016/j.jfma.2019.06.016.)
- Wang CY et al, “National survey of ABC (A1C, blood pressure, cholesterol) of Diabetes Health Promotion Institutes in Taiwan: 2002-2018”, J Formosan Med Assoc, 2018, Vol 117(11), pp 952–954. (DOI: 10.1016/j.jfma.2018.08.013.)
- Chu CH et al, “Trends in antidiabetic medical treatment from 2005 to 2014 in Taiwan”. J Formosan Med Assoc, 2019, Vol 118(2), pp S74-S82. (DOI: 10.1016/j.jfma.2019.06.001.)
- Chien MN et al, “Glycemic control and adherence to basal insulin therapy in Taiwanese patients with type 2 diabetes mellitus”. J Diabetes Inv, 2016, Vol 7(6), pp 881–888. (DOI: 10.1111/jdi.12532.)
- Sanofi (data on file).

