To Issue 152
Citation: “Product Showcase: CCBio’s Omega & Salus Pen Injectors”. ONdrugDelivery, Issue 152 (Oct 2023), pp 68–69.
THE ROLE OF PEN INJECTORS
According to the WHO’s definition, drug adherence refers to “the degree to which the person’s behaviour corresponds with the agreed recommendations from a healthcare provider”. When it comes to clinical treatment and managing chronic diseases, it is common for patients to experience a decline in their drug adherence, often due to the disease being asymptomatic or symptoms improving. This leads to prolonged illness, increased healthcare costs and increased disease complexity, creating a vicious cycle.
“There is clear evidence to suggest that self-injection is an effective strategy for improving medication adherence in gastrointestinal therapy.”
Currently, leading pharmaceutical companies provide clinical recommendations for the treatment and management of chronic diseases using drug delivery systems, such as pen injectors. There is clear evidence to suggest that self-injection is an effective strategy for improving medication adherence in gastrointestinal therapy, as these pen injectors, compared with traditional hospital-administered injections, reduce discomfort and enhance user-friendliness.1
Starting with a focused design and progressing through development to practical market cases, CCBio has developed a solution that balances performance, robustness and usability, drawing inspiration from the patient-centric usage patterns of pen injectors. CCBio offers two optimised pen injectors designed in response to the concerns of both pharmaceutical companies and patients – Omega and Salus. These two modern pen injectors from CCBio aim to provide pharmaceutical partners with a better choice.
OMEGA
CCBio’s Omega pen injector is a multidose injection device with a non-replaceable container (Figure 1) that has been successfully developed based on the needs from insights of pharmaceutical partners. Omega incorporates all the essential elements of a successful multidose injection device, for example, device length has been optimised in terms of user ergonomics, dexterity concerns, comfort and convenience for all use steps. Omega features a novel mechanical energy storage mechanism based on compression spring mechanics – rotating the dose selector recharges and stores energy every time the user dials in a dose setting.
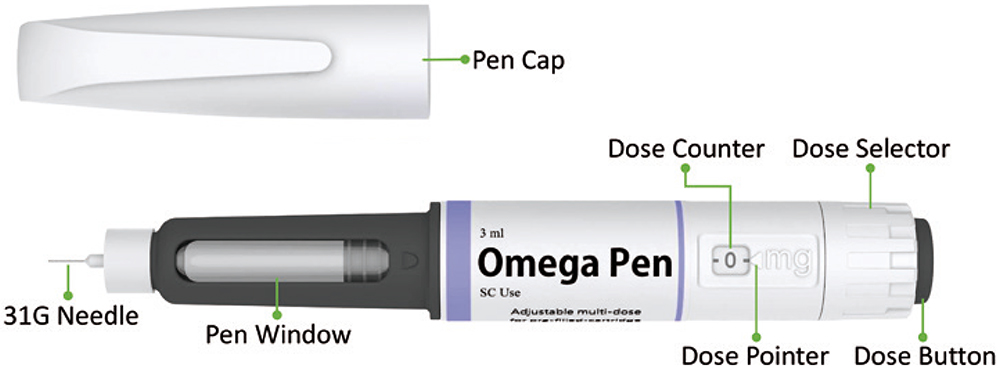
Figure 1: The Omega multidose pen injector.
SALUS
Likewise, CCBio’s Salus, a single-dose injection device with a non-replaceable container (Figure 2), has been developed based on key unmet needs communicated by pharmaceutical partners and incorporates all the essential elements of a successful autoinjector device.
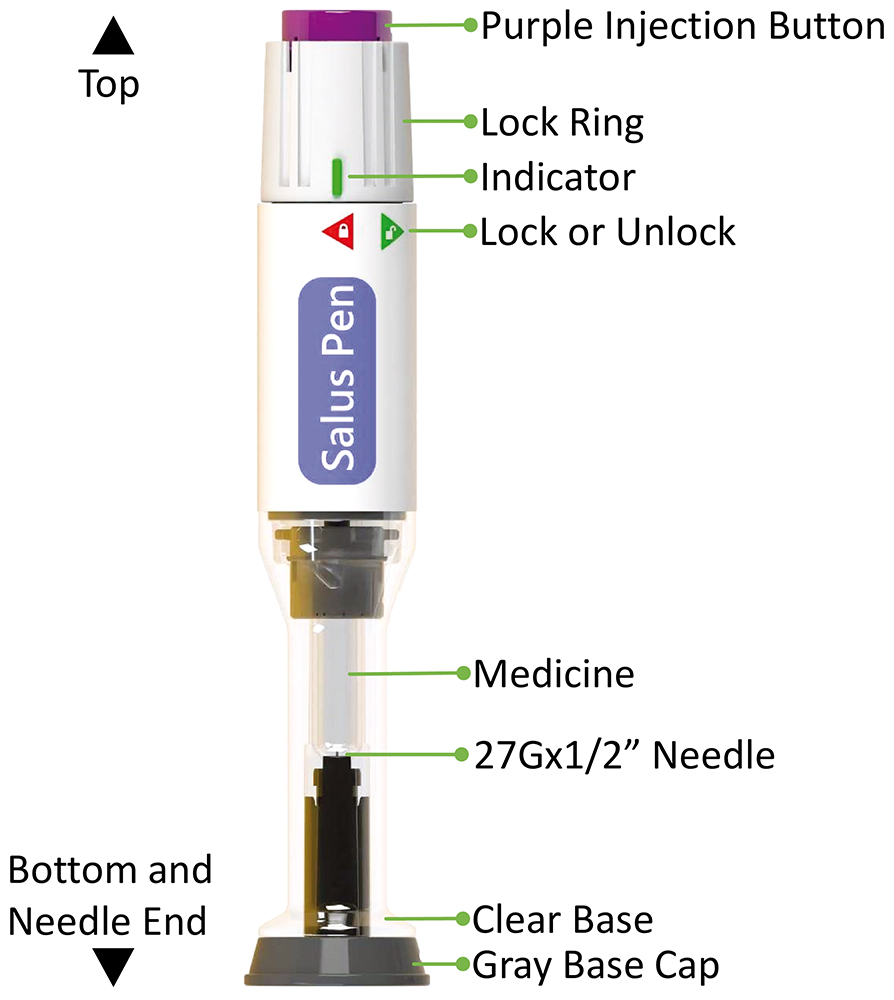
Figure 2: The Salus single-dose autoinjector.
CCBio leverages simple mechanical capabilities based on compression springs to facilitate one-time injection. After injection, the device provides passive needle protection, allowing patients with needle phobia to self-administer comfortably. The user-centric design includes an activation key for easy differentiation between pre-use and post-use. CCBio has also carefully designed the assembly process for integrating the drug-containing prefilled syringe into the autoinjector body, taking the pharmaceutical company’s perspective into consideration, making the assembly of Salus pen a straightforward two-step process. This minimises assembly errors and reduces the cost of assembly equipment.
CONCLUSION
In anticipation of the expiration of numerous drug patents within the next five years, particularly for combination product series, CCBio has identified a demand for a pen injector that can be paired with biosimilar drugs. Focusing on a response to this demand, CCBio has introduced two self-injection pen injectors, Omega and Salus (Table 1), with the hope of providing a valuable choice to customers in need.
| Property | Omega | Salus |
| Key Needs Addressed | Consistent with the usage model of the Ozempic® (semaglutide, Novo Nordisk) injection pen | Consistent with the usage model of the Trulicity® (dulaglutide, Eli Lilly) injection pen |
| Device Type | Mutidose injector | Autoinjector |
| Administration Route | Subcutaneous | Subcutaneous |
| Primary Container Format | 3 mL drug cartridge | 1 mL prefillable syringe; drug cartridge |
| Primary Container Material | Glass | Glass |
| Usage | Mutidose; disposable | Single dose; disposable |
| Dose Volume | 1.5 / 3 mL | 0.5 mL |
| Priming & Dose Setting | Priming or dose setting by the dose selector | N/A |
Table 1: Properties of the Omega and Salus autoinjectors.
To learn more about CCBio’s full portfolio of injection devices, which includes pens, autoinjectors and wearable injectors, visit: www.ccbio.com.tw/products.
REFERENCE
- Molina JT, Robeldillo JCL, Ruiz NC, “Potential Benefits of the Self-Administration of Subcutaneous Methotrexate with Autoinjector Devices for Patients: A Review”. Drug Healthc Patient Saf, 2021, Vol 13, pp 81–94.

