Citation: Moulin A, Kowalski L, “Compaction Simulation / Industrial Press Correlation: Two Case Studies. ONdrugDelivery Magazine, Issue 99 (Aug 2019), pp 24-27
Aline Moulin and Lucile Kowalski examine two case studies comparing compaction simulation with industrial compression.
Compaction simulation has been of scientific interest since the 1970s, with a first compression simulator described in 1976.1 The objective of compaction simulation is to be able to simulate industrial compression – that is, to be able to predict which parameters to apply on industrial equipment to obtain a tablet with the desired properties.
“ The objective of compaction simulation is to be able to simulate industrial compression – that is, to be able to predict which parameters to apply on industrial equipment to obtain a tablet with the desired properties.”
We have been involved in compaction simulation since 2016, with a STYL’One Evolution (Medelpharm, Beynost, France) compaction simulator. The choice of this equipment was driven by two key requirements. It had to be able to:
- Simulate industrial rotary presses
- Simulate complex compressions, such as multi-layer and tab-in-tab (also known as press coated tablets) which are the core Skyepharma development and industrialisation know-how.
Indeed, STYL’One Evolution has some characteristics that correspond well to our needs:
- It is equipped with two sensors on each punch thus allowing very accurate measurements of both tamping force and compression force in a very short time from 0 to 50 kN
- It simulates compression cycles of rotary industrial presses made possible by the fact that:
- You will use the same punches and dies as the ones you use on your industrial equipment
- The equipment mimics the kinetics of rotary presses, in terms of punches speed, penetration2 and dwell time3
- It also takes into account symmetric and non-symmetric punch penetration on your industrial equipment (Table 1)
- And finally, Medelpharm designed a specific feeding system for the introduction and centring of the core tablet during tab-in-tab compression. In the following paragraphs are two case studies of STYL’One / industrial equipment correlation established in our lab.
| Press | Type | Compression Symmetry | Punch with Fixed Stroke |
| SVIAC PR51 CM3 | Pilot multi-layer | Non-symmetrical | Upper punches |
| HATA HT AP LSU 3L | Industrial multi-layer | Non-symmetrical | Upper punches |
| FETTE P2100 | Industrial multi-layer | Symmetrical or non-symmetrical | Upper punches in non-symmetrical mode |
| Kilian S 250 M | Industrial press coater | Non-symmetrical | Upper punches |
Table 1: Different types of industrial presses of interest for our team.
CASE STUDY 1: GEOMATRIX® MULTI-LAYER TABLET
Objective
The objective of this case study was to be able to replace the early-stage prototyping step we usually perform on industrial equipment (SVIAC PR51 CM3) by prototyping on the STYL’One Evolution.
Geomatrix® multi layer technology allows sustained release profiles to be achieved. Regulating and extending drug release is advantageous in many ways:4,5
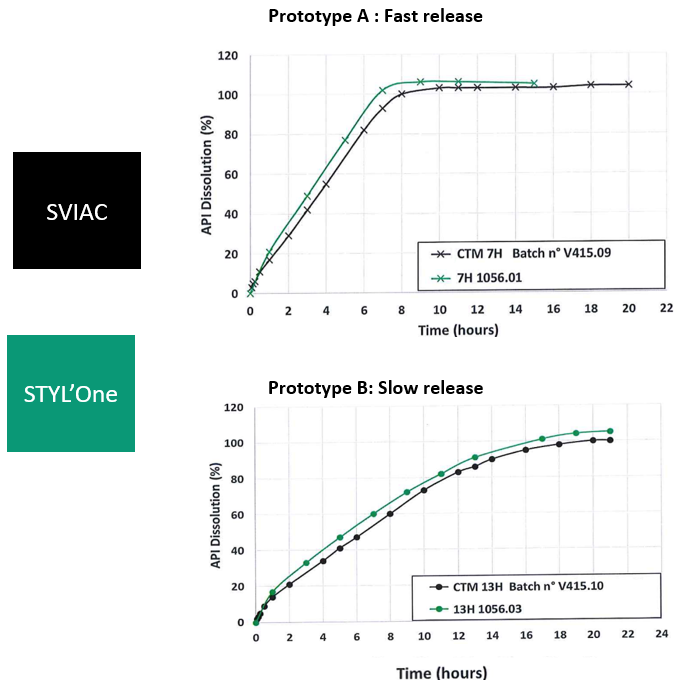
Figure 1: Dissolution profiles obtained for prototypes A and B manufactured with industrial equipment and with compaction simulator.
- Reduced fluctuations of the blood concentration of the active ingredient might result in decreased occurrence and severity of adverse effects
- Prolonging of the plasma concentration of drugs with short half-life also means reduced administration frequency, and improved patient compliance
- Benefits mentioned above are particularly important not only for patients themselves but also for clinicians and pharmaceutical scientists.
Methodology
The methodology was to demonstrate correlation between STYL’One and SVIAC PR51 CM3 on prototypes – to demonstrate that similar dissolution profile performance could be obtained for batches manufactured with SVIAC and with STYL’One equipment.
Results & Discussion
Two different prototypes were tested: prototype A (fast-release prototype) and prototype B (slow-release prototype). For each of them, the dissolution profiles are displayed in Figure 1, with two dissolution profiles for each of them:
- In black, the dissolution profile of the batch manufactured with the SVIAC PR51 CM3 equipment
- In green, the dissolution profile of the batch manufactured with the STYL’One Evolution compression simulator
We can observe that, for both prototypes, the black and green dissolution curves are similar (F2 > 50), thus demonstrating good correlation. Encouraged by these good results obtained on multi-layer tablets, we looked at even more complex oral solid dosage forms, i.e. press-coated tablets.
CASE STUDY 2: GEOCLOCK® TAB-IN-TAB/PRESS-COATED TABLET
Objective
This case study is about a tab-in-tab product, using Geoclock® technology, developed as a time-controlled pulsatile release formulation.6,7,8
Chronological disorders exhibit diurnal variations in their amplitudes owing to the circadian rhythm of the body (the body clock). Various chronological disorders – such as bronchial asthma, rheumatoid arthritis, variant/Prinzmental’s angina and hypertension – exhibit peak disturbance in the early morning when patients are asleep. Conventionally, such early-morning attacks are treated with bedtime administration of medicines in the form of either immediate release or extended release formulations. In such cases, although effect is needed only in the early morning, the drug is continuously released throughout the night; entailing higher doses to extend its effect up to the next morning, with some risks of side effects.
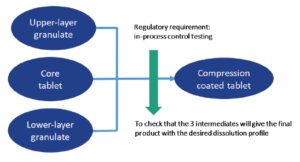
Figure 2: General scheme of the manufacturing process.
One alternative approach is the pulsatile drug delivery system that can restrict the release for a predetermined period and subsequently provide burst drug release to exhibit peak effect in the early morning. The most critical facet of a pulsatile release formulation is the lag time which can be engineered through different formulation strategies such as film coating or compression coating.
Administration of the proposed formulation is made at bedtime and will release the drug only after midnight to provide the effect in the early morning.The product of our case study presents a lag time, with a very narrow performance window:
- Sufficient lag time is needed to obtain the delayed release performance for middle-of-the-night delivery
- A too long lag time is to be avoided because of loss of bioavailability (API less absorbed in the colon).
Our objective was to demonstrate correlation between KILIAN S 250 M and STYL’One Evolution on this specific process.
Methodology
Figure 2 presents a high-level scheme of the process: manufacturing of the core tablet, of the upper-layer granulate and the lower-layer granulate, and final compression of the press-coated tablet. Given the very narrow performance window of this product, a press setting is performed to determine at which pressure the three intermediates will give the final product with the desired dissolution profile.
This setting is currently performed on an industrial KILIAN S 250 M press, and we wanted to see if it was possible to perform it on the STYL’One Evolution compression simulator. So a preliminary study was performed to compare lag-time dissolution performance between batches manufactured on both types of equipment.
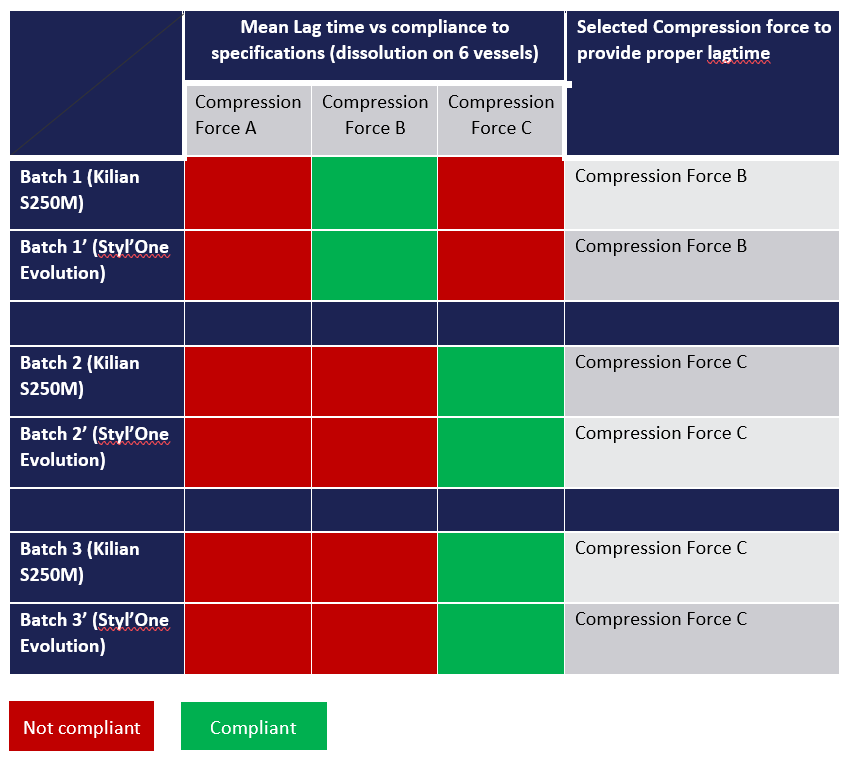
Figure 3: Comparison of the results obtained with Kilian and with STYL’One Evolution.
Results & Discussion
The comparison was performed with three different independent batches. For each of them, the setting was performed with Kilian equipment and with STYL’One Evolution equipment. Three different compression forces were tested – A, B and C – to determine if the resulting lag time was compliant with specifications.
The setting performed on STYL’One Evolution gave the same results as the one performed with KILIAN (Figure 3). That means the setting performed on STYL’One Evolution allows selection of compression pressure that, once applied on Killian S250, will give drug product with compliant lag time. Based on correlation observed in this case study, definition of the press-coating compression force required for industrial tablet press could be carried out on the STYL’One with minimal waste of resources.
CONCLUSION
Good correlations were established between industrial equipment and the STYL’One compaction simulator, on two different examples: multi-layer tablet and compression-coated tablet. Based on these results, we can move forward by using the compaction simulator at different stages all along the pharma development of a new project – early stage, scale-up, quality by design with design-of-experiment matrices performed at small scales, and even in-process control during commercial manufacturing.
REFERENCES
- Hunter B et al, “A high speed compression simulator”. J Pharm Pharmacol, 1976, Vol 28, Suppl 65P.
- Rippie E et al, “Viscoelastic stress / strain behavior of pharmaceutical tablets: analysis during unloading and postcompression periods”. J Pharm Sci, 1981, Vol 70 (5), pp 476-482.
- Qiu Y et al (Eds), “Developing Solid Oral Dosage Forms: Pharmaceutical Theory and Practice – 2nd Edition”. Published by Elsevier, Nov 28, 2016.
- Vavari G et al, “Matrix systems for oral drug delivery: formulations and drug release”. Drug Discovery Today: Technol, 2018, Vol 27, pp 71-80.
- Kuentz M et al, “Methodology of oral formulation selection in the pharmaceutical industry”. Eur J Pharm Sci, 2016, Vol 87, pp 136-163.
- Khan Z et al, “Drug delivery technologies for chronotherapeutic applications”. Pharm Dev Technol, 2009, Vol 14 (6), pp 602-612.
- Lin S-Y et al, “Current status and approaches to developing press-coated chronodelivery drug systems”. J Control Release, 2012, Vol 157 (3), pp 331-353.
- Patadia R et al, “Investigating critical effects of variegated lubricants, glidants and hydrophilic additives on lag time of press coated ethylcellulose tablets”. Pharm Dev Technol, 2016, Vol 21 (3), pp 302-310.

