Citation: Tunkel M, “Considerations for Device Selection in Parenteral Applications”. ONdrugDelivery, Issue 117 (Mar 2021), pp 66–69.
Mark Tunkel looks at the challenges involved in the selection of drug devices for combination products and the optimisation of the development process.
HOLISTIC DEVICE SELECTION AND USER-EXPERIENCE INNOVATION TO DRIVE DIFFERENTIATION
“While patient centricity is the foundation for developing a robust device selection plan and completion of a successful combination
product development initiative, developers need to remember there is a larger ecosystem that must be
considered to ensure success.”
The complexities involved in selecting the correct device for a combination product have never been more challenging. A convergence of trends, including significant growth in the global drug-development pipeline, the need for more complex delivery devices to address targeted applications and drug attributes, and increased migration of care from clinical to self-administration in home settings have driven demand for a wide range of solutions. This is coupled with a crowded competitive landscape in the biologics, biosimilars and generics segments, in which multiple competitors may be pursuing the same applications. This drives the need for differentiation wherever possible, across the entirety of the care continuum, well beyond solely the drug administration event, to supporting the broader patient and clinical journey. All these factors lead to a need for developers to adopt a holistic approach to device selection, which is focused on the entire combination product that spans development stages and requires specialised expertise at every step, and recognition of a variety of factors and influences that need to be considered to better serve patients, whose expectations are increasing. Nemera believes embracing this approach will lead to better near- and long-term decision making across the life cycle of drug products.
COMBINATION PRODUCT ECOSYSTEM
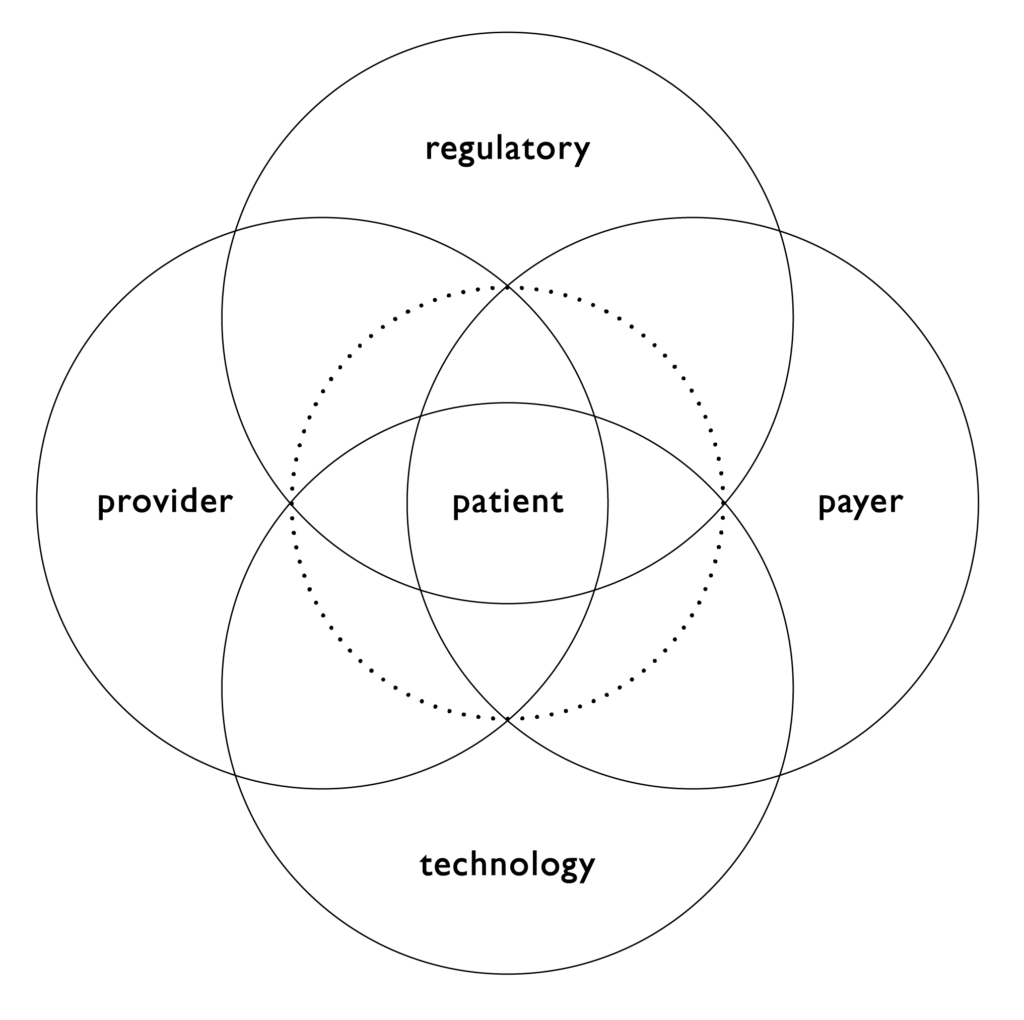
Figure 1. The combination product ecosystem.
While patient centricity is the foundation for developing a robust device selection plan and completion of a successful combination product development initiative, developers need to remember there is a larger ecosystem that must be considered to ensure success. This ecosystem includes providers, namely healthcare professionals, but also health systems; payers, in order to consider factors such as value-based care; and regulators, as the market for product introduction and the intended filing approach can significantly impact device selection approaches and development strategies. These factors then need to be considered within the available or emerging technology landscape. This broader understanding is critical in meeting the needs of patients and can impact how technology is selected, as well as early considerations around the impact of the other aspects of the ecosystem in development initiatives. Nemera believes that success will come from addressing these factors both strategically and tactically (Figure 1).
THE PATIENT AND CLINICAL STAKEHOLDER JOURNEY
With respect to patient centricity, at the earliest stages of establishing the functional requirements and user needs for a device, it is critical to fully understand the patient journey, as well as any related clinical processes, to ensure every decision considers their needs first. Understanding both this journey and interactions with the healthcare system and healthcare provider experience enables the capture of the complete process patients go through in managing their disease – both from a self-administration standpoint and from a longitudinal perspective – as they progress with their condition and treatment through the healthcare system and life stages.
“With respect to the device, decisions can be taken around assessing the technology landscape to identify existing IP or platforms that may be fit for the intended purposes related to function and drug product attributes.”

Figure 2. Contextual research provides a global understanding of the patient and clinical stakeholder experience in self-administration.
Nemera’s design research experts do this by primarily using a technique called “applied ethnography”. This method relies on a combination of interviews and in-context observations of practices, processes and experiences within the patient’s home or actual use environment (Figure 2). At this stage, potential applications are looked at broadly, that is beyond the administration event or solely complying with instructions for use as you might see in a human factors study. This can potentially start from when a patient is first diagnosed, to receiving their device, through the entire process of preparing, administering and disposal, and the times in between treatment so it can be understood how that process changes over time and how the frequency of administration may impact the patient experience. This gives the most natural view of the patient experience in relation to their environment, social/emotional contexts and all the other factors that influence use. As mentioned, it is equally important to gain an understanding of the experience of healthcare professionals, as well as considering this in relevant settings in clinical environments. This is of particular importance in applications where care is provided in both home and clinical environments, as well as a migration of care – such as an oncology ward, which has built-in support systems – to an environment of self-administration where clinical personnel are not present, and the burden of support falls to a family member or caregiver. These cases are also driven by migration in drug-delivery modality such as from intravenous to self-administered subcutaneous injection.
The outputs from these approaches include patient journey and clinical process maps, a robust understanding of prioritised user needs and values, and identification of pain points, which can be leveraged by cross functional development and commercial teams to consider possibilities for improving the patient and provider experience across all aspects of the journey to fully engage with patients beyond medication delivery (Figure 3).
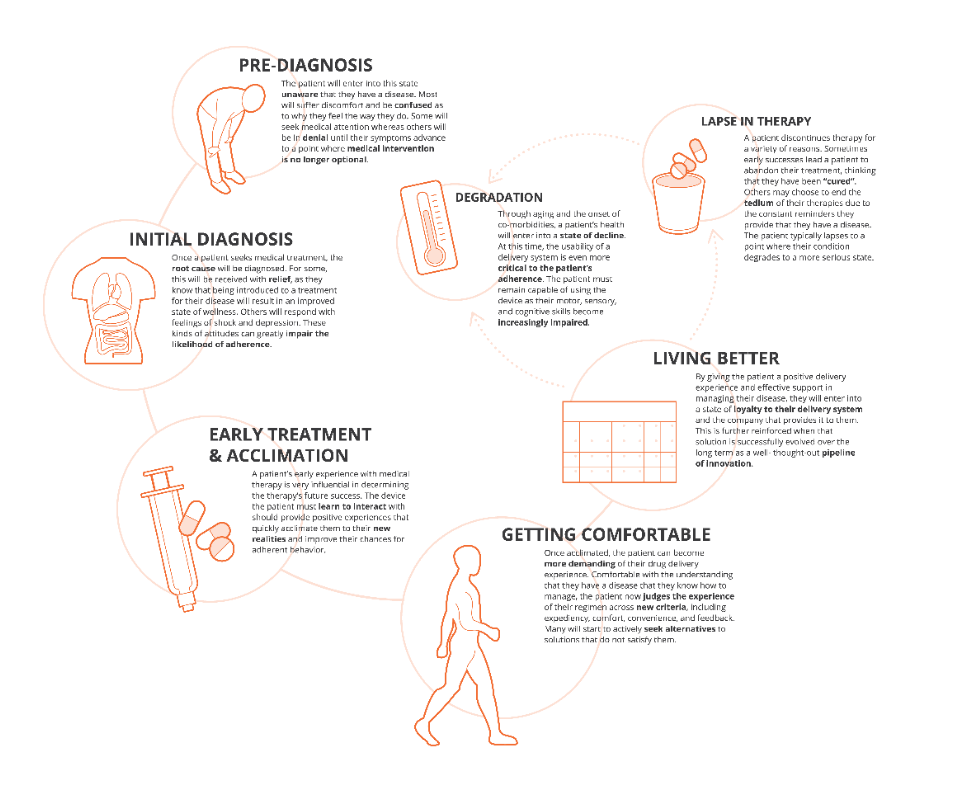
Figure 3: Key milestones along the patient journey and implications for delivery device design.
SYNTHESISING THE PATIENT JOURNEY AND ECOSYSTEM TO DRIVE DEVICE SELECTION
Having gained visibility of what the patient and stakeholder needs are, developers should consider how best to satisfy those needs as holistically as possible, while continuing to work with patients and clinical stakeholders prior to new product development processes. This requires synthesising the patient and stakeholder information with other ecosystem inputs into a device strategy roadmap, which can be used in several ways to make device selection decisions.
With respect to the device, decisions can be taken around assessing the technology landscape to identify existing intellectual property (IP) or platforms that may be fit for the intended purposes related to function and drug product attributes. This includes decisions around modality, such as autoinjector versus wearable injector, as well as variations within, if considering existing IP platforms. Early-stage lab and analytical activities may be used to assess any of this existing IP to ensure operational limits are sufficient for known user and functional requirements, as well as to assess compatibility with primary container solutions. This can help determine the need for customisation of existing IP. An example of this might be a modified form factor or affordance for user feedback on a pen or autoinjector, as well as refinement of operational aspects such as injection force to address targeted user groups.
If an existing technology or IP is not readily available, developers may choose to conduct early-stage technical concept generation and feasibility of new-to-the-world concepts/IP platforms while considering the ability of a concept to be scaled into commercial manufacturing.
In both instances, it is increasingly common to seek solutions that may be suitable across multiple drug products as part of a longer-term strategy to leverage a single device platform to drive efficiency in this manner. However, this brings the potential for a wide range of user and functional performance requirements, such as dose duration, frequency or patient population defining characteristics that need to be considered to ensure that targeted devices or concepts can be made to address specific requirements of each asset.
Regarding patient experience, it is useful to project future state-care models where devices are supported by digital health assets, training and other forms of engagement to address pain points and enhance the experience overall. This can help outline areas where partnerships with complementary providers may be necessary to address aspects of the model. Throughout this process, it is important to continue to work with patients and stakeholders to assess these options to ensure all needs are met. This provides confidence that the best decisions are being made at the earliest stages before resource-intensive development activities.
OPTIMISING THE DEVELOPMENT PROCESS AND “SURROUNDING THE DEVICE” FOR DIFFERENTIATION
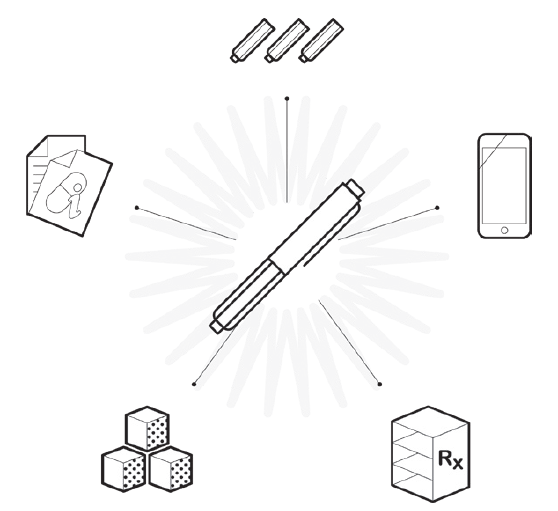
Figure 4. Optimising the development process and “surrounding the device” for differentiation is critical.
Beyond making device selection decisions, it is important to use this foundation to consider the product development process including differentiation of the user experience, support of clinical trials from both a usability and device supply perspective that the broader capabilities at Nemera can support, and life-cycle management to extend the value of devices through enhancements based on market surveillance or other inputs, as competition may emerge. Ideally, this will all feed back into the definition and development process for next-generation devices (Figure 4).
It is key to drive user experience differentiation and to optimise the selected device for the target drug product. To achieve this, human factors and patient experience activities must be integrated for a successful drug-device combination product development process. It is incumbent on developers to ensure that the selected device, in combination with the drug, is appropriate, safe and effective for the target population. This also extends to executing the earlier-stage inputs to optimise the patient experience to create competitive differentiation and to ensure adherence and engagement with patients and clinical stakeholders. Using the foundation that established them defines the right blend of complementary user-experience elements to achieve these goals.
Good examples of this approach are Nemera’s recently acquired pen injector portfolio and large-volume wearable concept under development. These devices are of interest to customers in the biologic, biosimilar or generic markets, where, in many cases, competitors are targeting the same reference drug or devices, and differentiation wherever possible is critical. Using the foundation discussed earlier, Nemera can support the entire combination product development process and help its customers in “surrounding the device” in every way possible including:
- Addressing the defined user groups/populations and early-use related risk analysis activities to define the human factors and usability programme necessary for the intended regulatory/filing strategy and identified clinical risks, through conducting formative and summative usability testing globally for all aspects of the device and supporting assets in alignment with the human factors programme definition, through human factors engineering report documentation for use in regulatory submissions.
- Developing instructions for use, value-added packaging specific to the application, which may include leveraging digital health related add-ons to support patient engagement/adherence, as well as extend the value of a device platform.
- Development of a formal training programme, including device training devices into the patient services model to create commercial differentiation. Nemera’s partnership with Noble (Orlando, FL, US) Safe’n’Sound® sound device is an example of this approach.
- Leveraging Nemera’s clinical and commercial manufacturing regardless of device modality.
- Programme management excellence to ensure all elements of the programme are integrated to drive efficiency and mitigate any programme risks proactively as a single partner for development of the combination product.
In summary, the combination of Nemera’s strong IP platforms, development services and manufacturing services allows customers to achieve the outcome of a successful regulatory submission and commercial launch of safe, effective and differentiated combination products with a single partner who can manage all of the aspects critical for success.
Previous article
PLATFORM TRAINING SOLUTIONS ADD VALUE FOR PHARMACEUTICAL COMPANIESNext article
ROOM FOR INNOVATION?
