Citation: Birkhoff M, “Delivering on the Growing Need for Topical Preservative-Free Ophthalmic Treatments”. ONdrugDelivery Magazine, Issue 104 (Jan 2020), pp 8-12
Matthias Birkhoff provides an overview of existing ophthalmic delivery technologies and progress made within topically applied medications – and introduces a new market standard for testing microbial integrity in preservative-free formulation.
Ophthalmic therapeutics, along with more general vision care, are vital components of the global healthcare landscape due to their very demonstrable impact on the quality of life for patients with ocular disorders.
As such, ophthalmic drugs represent a significant part of the global pharmaceutical market, with sales of more than US$24 billion (£18.5 billion) annually in 2018. This figure continues to grow across the world as there is an increasing prevalence of eye disorders, particularly among the elderly population. Retinal disorders such as age-related macular degeneration (AMD), diabetic retinopathy (DR) and diseases such as presbyopia, dry eye syndrome, glaucoma, conjunctivitis, red eye and uveitis are driving growth in ophthalmic treatment needs. In addition, the general increase in consumer demands and spending power in emerging markets such as Brazil, Russia, China and India also contributes to a sales growth.
WE NEED TO KEEP AN EYE ON THE CHALLENGES
The success of any drug discovery and development programme requires true collaboration between formulation scientists and device manufacturers. This collegiate approach is no different in ophthalmic drug delivery. Indeed, some may conceive it as even more critical to success than in any other delivery route, particularly when trying to address unmet needs in the treatment of eye disease.
There are, however, some challenges associated with ophthalmic drug delivery. Undoubtedly, the primary way to administer eye medications remains by means of topical drops. Even today, novel intraocular pressure (IOP) reducing medications are administered as topical eye drops. But, at the same time, development of sustained release delivery technologies for treatment of ophthalmic diseases has made substantial progress and is considered to increase patient compliance and achieve a higher therapeutic benefit.
Looking at retinal disorders, almost all available treatments require invasive procedures since none of the currently available therapeutic alternatives offer options for topical administration. However, it seems fair to assume that patients prefer to take drops rather than receive injections into their eyes. This all makes the “traditional” way of administering ophthalmic medications worth considering, even in the future. This method does, of course, rely heavily on the patient’s willingness and ability to administer the product and the perceived side effects associated with such administration.
Also the increasing cost pressure from payers needs to be taken into consideration. Healthcare systems around the world are very different, with some patients bearing all the costs and others none or just a fraction. In particular, the latter ones will continuously look for costing improvements while the ever-ageing population continues to grow, as does demand for age-related ophthalmic treatments. With these aspects of cost effectiveness and security-of-supply in mind, it is fair to conclude that it will be very difficult to rely entirely on invasive methods and treat patients properly without topical ophthalmic medications. Given these considerations, it makes sense for innovative pharma companies to develop topical administrations in a way that patients and consumers can embrace.
WHILE NECESSARY FOR SAFETY AND STABILITY, PRESERVATIVES PRESENT PROBLEMS
Figure 1: Taflotan (tafluprost) as marketed by Santen (Image courtesy Santen), and Restasis (cyclosporine) as marketed by Allergan (Image courtesy Allergan), both of which use Aptar Pharma’s Ophthalmic Squeeze Dispenser platform.
The use of preservatives in eye-care formulations – both prescription and over-the-counter products – has come into sharp focus in the past few years. Preserved topical medications have been the mainstay of treatments for patients prescribed with varying lengths of regimen. In most of the ophthalmic medications, preservatives are therefore required to prevent the growth of microbial contaminants, mitigate against biodegradation and keep the drug product stable and safe over time.
However, historically used preservatives, such as benzalkonium chloride, are known to have tolerability issues in patients. Indeed, some preservatives themselves present a risk, with clinical data1 suggesting that continuous exposure to certain preservatives can result in various side effects such as ocular surface changes, ocular discomfort, tear film instability, conjunctival inflammation, subconjunctival fibrosis, epithelial apoptosis, corneal surface impairment, subclinical inflammation and even the potential risk of failure in further surgical interventions. Such adverse reactions can have an impact on patient adherence – especially in chronic diseases, like glaucoma, which require lifelong therapy with topical treatments for the eyes.
“The Ophthalmic Squeeze Dispenser’s unique safety profile contributes to the fact that it remains the only FDA-reviewed multi dose system for unpreserved ophthalmic formulations.”
DEVELOPING CHALLENGING TEST METHODS
A formulation containing no preservatives has its own challenges too, of course, and requires specific measures to ensure sterility, microbiological integrity and, therefore, patient safety. The key question in a preservative-free, multi-dose (PFMD) device must be how the pharma manufacturer can deliver enough evidence to show that, even under highly challenging conditions, microbiological integrity is maintained during shelf life and in use.
In the absence of guidelines regarding multi-dose containers for unpreserved ophthalmic preparations, test methods proving a microbial barrier function need to be carefully selected. However, there has been much progress in test methods since their introduction in the 1990s for the first PFMD systems, and Aptar Pharma strongly believes its recently published method TSIT 2.0 (tip-seal integrity test) is as close to a universal standard as the industry has right now.
Initial tests were developed to show safety of systems that contained silver ions in the plastic material of the dispensing system. The so-called Wiedemann2 test was developed according to the particularities of such systems, which release the abovementioned silver ions into the formulation. The acceptance criteria are a low microbial burden of the delivered dose and no contamination of the bottle.
In 2004, a tip-seal integrity test was developed using the same challenge germs but adding more challenging handling conditions.3 This test has been reviewed by agencies such as the US FDA and has enabled, among others, the launch of Allergan’s Restasis MultiDose in 2016 – the first FDA-approved prescription medication using a PFMD eye dropper, Aptar Pharma’s Ophthalmic Squeeze Dispenser (OSD). In 2019, Santen’s prescription drug Taflotan/Saflutan for the reduction of elevated IOP in open angle glaucoma and ocular hypertension was approved in 26 countries in Europe, again using the Aptar Pharma OSD (Figure 1).
Most recently, the TSIT method was further developed to align it more closely with the preservative-free tests described in the US Pharmacopeia (USP) <51> and European Pharmacopoeia (EP) 5.1.3.4 Aptar Pharma developed this method in order to provide a reasonable and standardised challenge procedure for PFMD-systems (Figure 2).
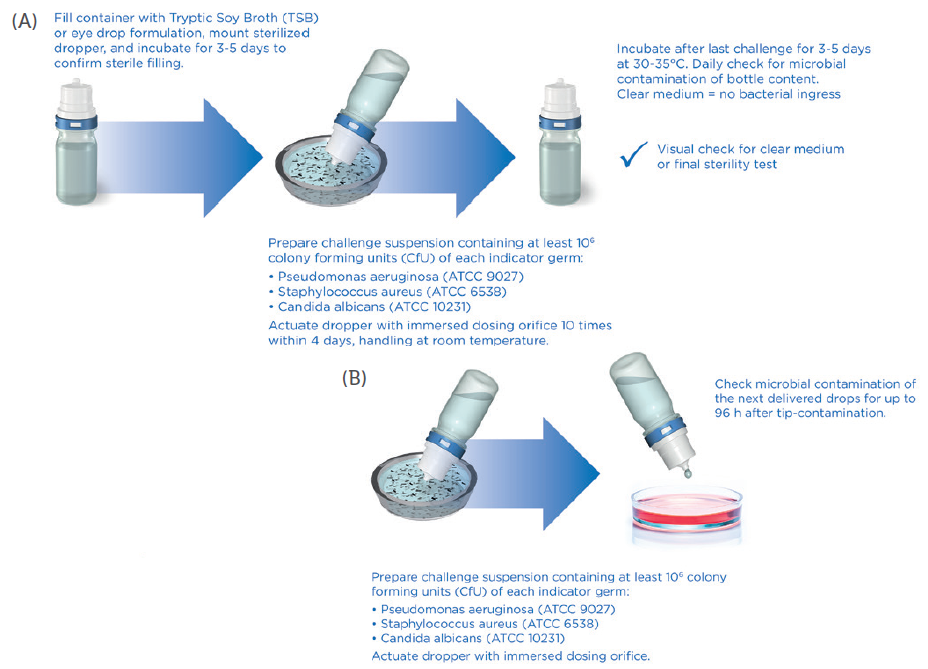
Figure 2: New microbiological challenge test (TSIT 2.0) as performed by Aptar Pharma, showing the principle of the TSIT 2.0 (A), and a test to check potential contamination of next dose (B).
Today, the TSIT 2.0 procedure provides a significant challenge for PFMD devices as it uses a relevant challenge scenario. Combined with an analysis of the microbial properties of the formulation (growth promoting, growth inhibiting or even bacteriostatic), as well as including two more germs, this method is well suited for the risk evaluation of new PFMD delivery systems. It indicates, using transparent, science-led data, that patients, physicians and the regulatory authorities can be certain about the safety of tested preservative-free ophthalmic medications (Table 1).
| Wiedemann Test | Established Aptar TSIT | TSIT 2.0 | |
| Rationale for test | Test was developed and optimised for Comod® and 3K® System (1993-94) |
Aptar TSIT adapted ~2004
to APF nasal spray pump
|
Request from authorities (e.g. BfArM) for broader challenge based on antimicrobial effectiveness testing EP 5.1.3 USP <51>, <771> |
| Test medium | Physiological saline | Growth medium | Growth medium, tryptic soy broth (TSB) |
| Indicator germs | Pseudomonas aeruginosa ATCC 9027 >106 CfU/mL |
Pseudomonas aeruginosa ATCC 9027 >107 CfU/mL |
Pseudomonas aeruginosa, ATCC 9027 Staphylococcus aureus ATCC 6538 Candida albicans ATCC 10231 At least 106 CfU/mL for each germ |
| Challenge procedure | Tip submersed in challenge suspension, then removed and actuated, 8 times within 4 days |
Tip submersed in challenge suspension and actuated, 10 times within 5 days | Tip submersed in challenge suspension and actuated, 10 times within 4 days |
| Incubation temperature | During challenge period ambient temperature, afterwards at 32ºC | Whole test period at 32ºC |
During challenge period ambient temperature (20-25ºC), afterwards at 32ºC |
| Parameters analysed | Analysis of spray and container content |
Analysis of spray and container content |
Only analysis of container content (other parameters may be included on request) |
Table 1: Test procedures for PFMD systems.
TSIT 2.0 HELPS FACILITATE MARKET ACCESS
Aptar Pharma’s US FDA- and German BfArM-reviewed Ophthalmic Squeeze Dispenser is a multi-dose technology designed specifically for preservative-free ophthalmic formulations. Suitable for a wide range of viscosities, it offers pharma partners unrivalled microbiological safety, which is why it leads the world with close to 250 market references in both prescription medications and consumer products. The Ophthalmic Squeeze Dispenser’s unique safety profile contributes to the fact that it remains the only FDA-reviewed multi-dose system for unpreserved ophthalmic formulations.
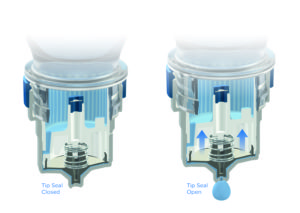
Figure 3: Aptar Pharma’s proprietary, purely mechanic tip-seal mechanism used in the Ophthalmic Squeeze Dispenser.
The Ophthalmic Squeeze Dispenser is a purely mechanical system. It does not contain any questionable additives that increase the complexity of approval processes or lead to constraints within the patient population. The tip of the orifice is protected with a sealing mechanism that – with proven reliability – avoids any microbiological ingress, while the air which is required to equilibrate the system passes a 0.2 μm filter membrane that is subject to 100% online inspection during manufacturing (Figures 3 and 4).
MULTI-DOSE, PRESERVATIVE-FREE DEVICES ADDRESSING UNMET NEEDS
Retinal disorders, such as diabetic macular oedema, are leading causes of blindness. In fact, the WHO estimates that 11% of the 347 million people suffering from diabetes also suffer from diabetic retinopathy. The gold standard treatment for this and other retinal diseases is currently anti-VEGF drugs which require frequent intravitreal injections. Obviously, this is an expensive strategy, and recent data shows that compliance with such therapies is not ideal.5

Figure 4: Close to 250 prescription medications and consumer products have been successfully launched with Aptar Pharma’s Ophthalmic Squeeze Dispenser.
Formulation scientists around the world are working on ideas to allow drugs to penetrate corneal and scleral structures without invasive procedures. The ultimate goal is to have anti-VEGF drugs as topically administered drops. Such formulations would clearly benefit from being preservative-free, as they would be for long-term treatments.
A SAFE, STABLE & SUSTAINABLE OPTION
What about single-use vials – also known as blow, fill and seal (BFS) technology? Before PFMDs were developed, BFS products were the only option for preservative-free ophthalmic formulations for topical treatments. Ophthalmic preparations can be filled and administered without the use of preservatives, and with a single use the risk of contamination is extremely low, provided neither patients nor consumers try to “stretch” the use of their vials, i.e. keep them for multiple use. The internet is full of instructions and “competitions” about how long one can use a single dose vial. Such strategies certainly do not contribute to the safety of ophthalmic medications.
Another challenge with single-dose applications is the pure amount of packaging they generate. BFS packs require a great deal of plastic and paper, as well as complex aluminium-based laminates used for pouching the single dose units – creating an additional environmental burden, both in terms of manufacturing and recycling. Proprietary research conducted by Aptar Pharma shows its Ophthalmic Squeeze Dispenser 10 mL multi-dose device generates a significantly smaller CO2 footprint than the equivalent in single-dose systems.
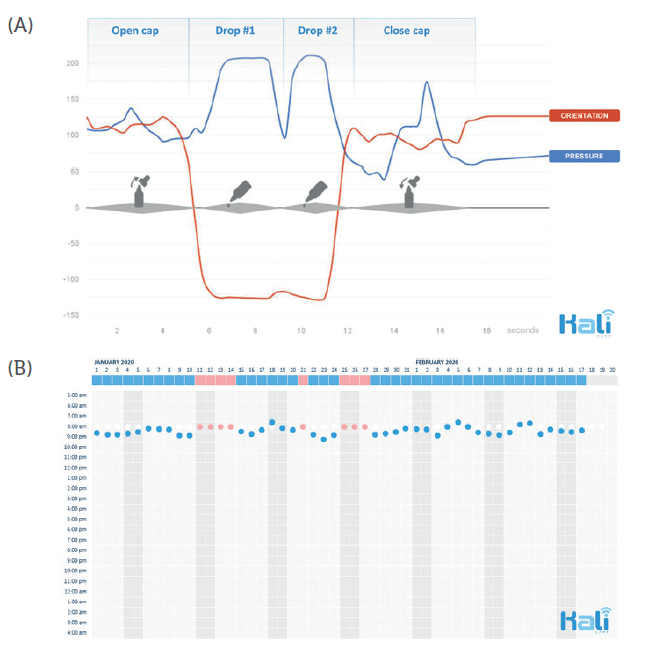
Figure 5: Typical motion sequence of administering eye drops, recorded with
Kali Care’s sensor technology (A). Collection of objective real-time data results
in reliable adherence charts (B).
BETTER CONNECTED DEVICES ENABLE BETTER PATIENT OUTCOMES
The final element in the compliance trinity is the increased use of connectivity. With soaring clinical trial costs and complexity, the biopharma industry is constantly seeking new approaches to improve efficiency and productivity. Aptar Pharma has teamed up with Kali Care (Mountain View, CA, US) to create a digital monitoring system for ophthalmic medications that will help reduce the costs and complexity of ophthalmic clinical trials.
This revolutionary sensor technology allows clinicians to replace adherence assumptions in clinical trials with collected real-time patient data that can be automatically integrated. This can result in shorter and more efficient clinical trials. Such an automatically generated adherence score provides critical information for explaining the incongruity between recommended treatment and actual treatment outcomes (Figure 5).
Various clinical trials have reported average adherence rates of only 43-78% among patients receiving treatment for chronic conditions.6 In clinical practice, the ability to see the medication-adherence score of patients with glaucoma is a powerful tool for ophthalmologists. In a very short time, we believe we will see devices optimised for orientation and pressure.
Aptar Pharma has been setting the standard for the drug delivery industry for decades. This collaboration with Kali Care once more underlines the company’s continuous efforts in breaking new ground in innovative healthcare technologies.
REFERENCES
- Baudoiun C, et al, “Preservatives in eyedrops: the good, the bad and the ugly”. Prog Retin Eye Res, 2010, Vol 29(4), pp 312-334.
- Bagel S, Wiedemann B, “Nasensprays ohne Konservierung”. Deutsche Apotheker Zeitug, 1999, Vol 46, p 48.
- Bommer R, Kern J, et al, “Preservative-Free Nasal Drug-Delivery Systems”. Med Device Technol, 2004, Vol 5(9), pp 14–6, 18.
- Marx D, Bartsch T, Wohnhas R, Zwisler W, “Microbial integrity test for preservative-free multidose eyedroppers or nasal spray pumps”. Pharmind, 2019, Vol 9, p 1247.
- Polat O et al, “Factors Affecting Compliance to Intravitreal Anti-Vascular Endothelial Growth Factor Therapy in Patients with Age-Related Macular Degeneration”. Turk J Ophthalmol, 2017, Vol 47(4), pp 205–210.
- Osterberg L, Blaschke T, “Adherence to Medication”. N Engl J Med, 2005, Vol 353, pp 487-497.

