Citation: Bogrash A, “Designing Drug Delivery Devices Through Collaborative Usability Testing”. ONdrugDelivery, Issue 124 (Sep 2021), pp 52–56.
Alice Bogrash examines how the company uses human factors studies to design drug delivery devices that respond to user needs, while providing a competitive advantage for pharmaceutical companies looking to introduce innovative combination products to the market.
“The wearable injectors that are expected to win strong market share will be those that are reliable, comfortable and easiest for the patient to use.”
Human factors – or “usability engineering” – is the science focused on the interaction between people and devices. Specifically, human factors is a key focus for medical device manufacturers, as they seek not only to optimise their device design through iterative user testing sessions but also to comply with regulators’ mandatory requirements and guidelines throughout the device development process.
The purpose of these guidelines, which have been developed, published and updated over recent years, is to assist medical device manufacturers with following “appropriate human factors and usability engineering processes to maximise the likelihood that new medical devices will be safe and effective for the intended users, uses and use environments.”1 Usability testing is an important aspect of any medical device design process but, when looking at devices used in home-care settings, usability engineering’s importance is further highlighted.
One of the primary purposes of wearable drug delivery devices (Figure 1) is to simplify the process of self-administration for patients, making home-care treatments more feasible. With a strong preference for “hospital at home” and patient demands for greater involvement in the care process, wearable drug delivery devices provide a solution for both healthcare providers and patients, increasing patient throughput in the clinical setting while also enhancing the user experience for patients, enabling them to receive their prescribed therapy in the comfort of their own home. The challenge is making wearable injectors that are easy to use and intuitive enough that a wide range of use groups and patient populations can use them, regardless of age, tech-savviness, dexterity and other factors generally influenced by the specific indication the drug product is looking to address.

Figure 1: The Sorrel wearable injector.
Although there are not many wearable injectors on the market as of yet – or even too many in clinical stages – they differ from each other significantly in terms of how the device is to be used; the number of steps they require to be activated, the method by which the drug is filled into the device, the reusable versus disposable aspects of each device and so on.
The wearable injectors that are expected to win strong market share will be those that are reliable, comfortable and easiest for the patient to use. It is therefore essential to examine the key human factors that must be considered throughout the development of self-administration devices – from basic design to final commercial product – to ensure that they provide a safe, intuitive and seamless user experience, empowering users to take control of their health, all in a home-care setting.
HUMAN FACTORS TESTING FROM INITIAL DESIGN
The process of human factors testing is, and indeed must be, an iterative one. From the initial design stage, it is imperative to eliminate all use-related risks via a robust and comprehensive risk analysis and mitigation process. A multidisciplinary evaluation will reveal any design issues that must be addressed before moving on to the first device prototype for further in-house testing and evaluation.
At Eitan Medical, each product line has its own in-house team for human factors and usability engineering. Whether they be wearable devices intended for self-administration or infusion pumps intended for both hospital and home-care use, a dedicated product management team has specific expertise and experience with human factors. That said, usability and human factors testing are not activities managed solely within the company, as the Eitan Medical human factors team partners externally with pharmaceutical companies and third-party vendors to support, plan and execute human factors studies throughout the product development lifecycle.
PLATFORM APPROACH FOR USABILITY TESTING
For the Sorrel wearable platform, it is important to differentiate between the generic Sorrel-branded platform devices and the specific device configurations customised as part of a partner-specific development programme. As part of the initial design and development of the Sorrel platform, testing was conducted on a wide variety of key aspects of device usability. These studies were conducted primarily on a general population of test subjects, with a wide range of ages, ethnicities, body mass indices (BMIs) and health conditions.
When Eitan Medical partners with a pharmaceutical company, the project is focused on a specific drug product, resulting in a clinical, then commercial, drug/biologic-device combination product. In such a case, the pharma partner is generally responsible for testing the customised Sorrel device for the specific and relevant patient population, use case and use environment, with the Eitan Medical human factors team supporting, or at times even running parallel studies, to provide a robust set of usability testing data as an output.
“From the initial design stage, it is imperative to eliminate all use-related risks via a robust and comprehensive risk analysis and mitigation process.”
The combined human factors team, with representatives from both the device and the pharma side, is an integrated unit bringing together the best of both worlds – the knowledge and experience from the pharma world with a specific indication and patient population on the one hand, and the device expertise on the other. The full set of human factors studies conducted throughout a joint programme ranges from smaller internal studies to local medium-sized studies to larger studies conducted in a variety of geographies run by third-party human factors firms.
DESIGN FLEXIBILITY TO ACCOMMODATE DIFFERENT USE CASES AND PATIENT POPULATIONS
The Sorrel wearable injectors were developed with inherent flexibility, as part of an overarching platform approach. What this means is that, as part of a molecule-specific development project, a Sorrel device can be customised and optimised for a specific drug product, use case and patient population (Figure 2).
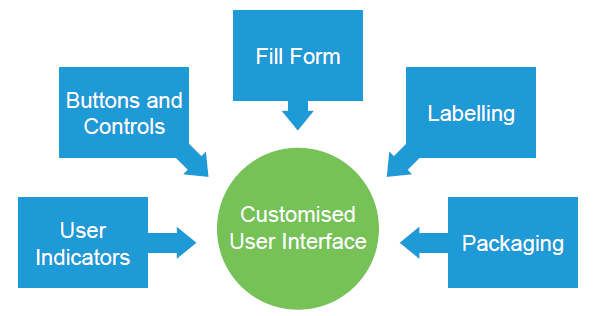
Figure 2: Customised features of a smart wearable drug delivery device.
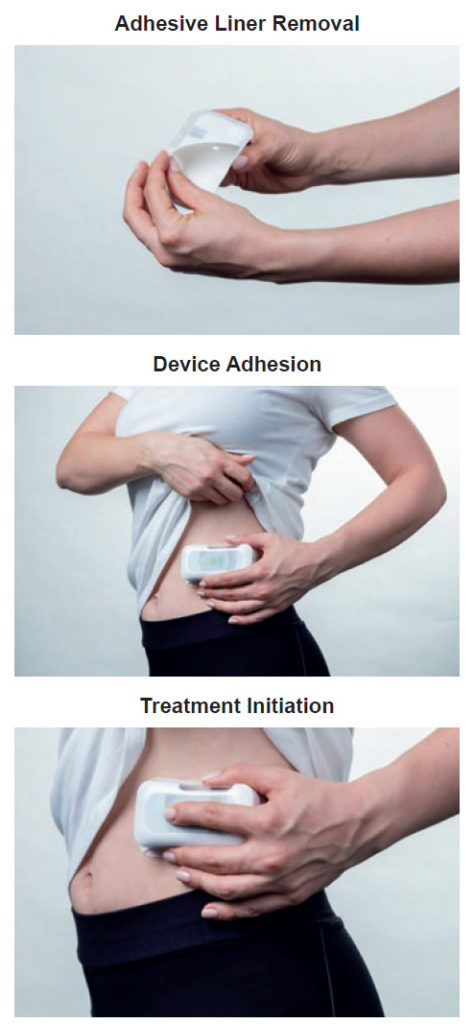
Figure 3: Critical use steps that may require dedicated indication sequencing.
These customised features include several aspects affecting the user’s interaction with the final combination product:
- User Indicators: For self-administration devices, indicators are essential for guiding the user through their drug delivery experience – providing the user with positive feedback throughout the treatment and alerting them if there is an issue. Indicators are important in that they point out the critical steps for using the device, as outlined during the user risk analysis (Figure 3). For example, if it is imperative that the user comprehends when the treatment has started, an indicator will be designed to signal that point in time to the user. The Sorrel devices include audio, visual and tactile feedback, which can be easily customised and quickly tested, as the device is software controlled. This means that not only can the sequence and duration of each indication be customised but it can easily be changed between one human factors study iteration and the next – allowing for ideal comparison throughout testing and quick optimisation of user indications. Further customisation can be performed for specific patient populations – increasing the strength of the light indicators or buzzer sounds for those suffering from visual or aural impairments, respectively.
- Buttons and Controls: Drug delivery devices may require some form of push button or switch, whether to activate the device, initiate drug delivery, administer a bolus or pause and resume treatment. The Sorrel devices include a software-controlled hard key that can be configured for one or several actions. As an example, a long press can mean treatment initiation, while two consecutive button presses may mean a bolus administration. Alternatively, with the use of the proprietary on-body sensing mechanism – consisting of a trigger and supporting algorithm – the device can forgo a button altogether and the treatment can begin automatically once the device has been adhered to the skin properly. These decisions are made on a case-by-case basis, as part of the joint usability evaluation between the Eitan Medical team and the pharmaceutical partner.

Figure 4: For a manually filled device, emphasis must be placed on testing the filling or loading process.
- Fill From: A key parameter affecting the user experience in drug delivery devices is the way in which the drug is filled or loaded into the device. A drug-administration process that entails the least number of user steps will inevitably reduce the probability of use errors, as each additional step is a potential point of failure. Not only does the drug filling or loading aspect of the combination product need to be assessed as part of the overall human factors testing plan (Figure 4), but the device design should be optimised in a way that the overall steps for the user are kept to a minimum. The Sorrel platform includes unique technology solutions to enable a prefilled and preloaded device configuration, whether using a cartridge or vial, enabling the ideal user interface where – essentially as simple as using a plaster – all the user needs to do is peel the adhesive liner and stick the device on their body.
- Labelling: Effective labelling is a key element in directing patients towards safe and effective use of a device, while mitigating any potential use-related hazards. Labelling includes the information on the device itself, as well as labelling on the packaging of each device and the overall combination product, together with the instructions for use. These graphics and guides are jointly developed between the device manufacturer and the pharmaceutical partner and are included in the iterative human factors review process. The general approach at Eitan Medical is to reduce dependency on the user reading the instructions for use by using graphics and text on the device itself, on the adhesive liner and on the packaging as an effective way of guiding the user through their use of the Sorrel devices.
- Packaging: As with labelling, significant investigation must be conducted into the design of the device packaging. Key considerations will include the user experience when opening packaging, the ease with which users can extract the device from the package, and the packaging design’s ability to protect the device integrity in various conditions and during transportation.
USER ACCEPTANCE OF NEW DEVICE CONCEPTS AND DESIGNS
Not only can specific device features be tested, analysed and optimised through human factors testing, but similar studies can be used as a way of initial assessment of new device concepts. Groups of individuals, representing the target patient population demographics, can be interviewed to understand their acceptance of a new product, concept, feature or design.
For example, before initiating a study moving a treatment from hospital based intravenous infusion to at-home subcutaneous administration, potential users can be asked if they believe a wearable injector is something they can manage on their own – or if they would prefer having a healthcare professional present during their injection. Another question that can be targeted as part of a user preference study is if potential users would accept a 50 mL on-body drug delivery device as an alternative to several lower volume injections or a trip to the clinic.
These types of studies are regularly conducted at the start of joint ventures between Eitan Medical and pharmaceutical companies partnering with the Sorrel platform, as a way to receive early feedback from user groups – reducing risk and increasing the overall probability of success in a development project. Over the years, Eitan Medical’s team has gained valuable insight throughout user preference studies as a way of determining areas of focus for the company’s product pipeline.
“Not only can specific device features be tested, analysed and optimised through human factors testing, but similar studies can be used as a way of initial assessment of new device concepts.”
DESIGNING WEARABLES TO HELP PHARMA COMPANIES ACHIEVE THEIR COMMERCIAL GOALS
Pharmaceutical companies today seek to provide more value to their customers, with friendly devices that enable at-home administration of large-volume and high-viscosity medications. They are looking for partners that are able to support this goal, with innovative device technologies that enable patient self-administration. Eitan Medical’s pharmaceutical solutions business unit aims to help pharmaceutical companies appeal to the largest possible demographic and make the devices as easy to use as possible, de-risking market entry (Figure 5).
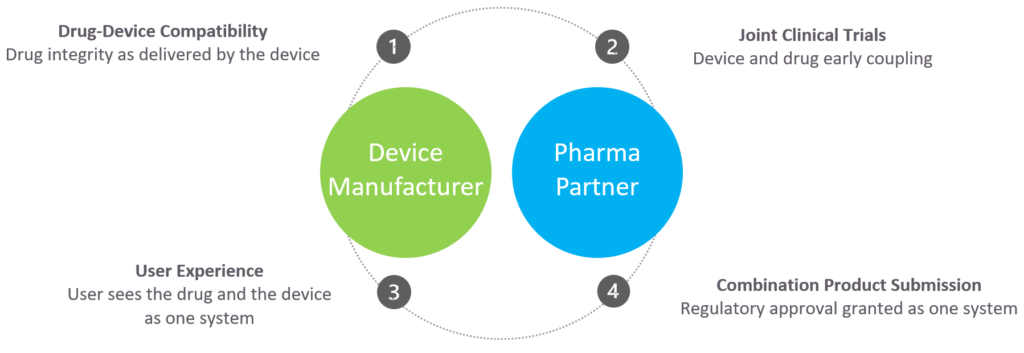
Figure 5: Collaborative approach throughout device development.
Designing a wearable drug delivery device with the patient in mind from the start, together with proven design capabilities, flexible platforms and a collaborative approach throughout human factors testing, ensures that the final device will be optimised from a usability perspective. This can, in turn, have a positive impact on adherence to treatment regimens, ensuring users receive medication in a safe, easy to use way, delivering competitive advantage, and helping both patients and pharma providers.
REFERENCE
- “Guidance for Industry: Applying Human Factors and Usability Engineering to Medical Devices”. US FDA, Feb 2016.


