To Issue 152
Citation: Fong A, “Device Interchangeability Study of Three-Step and Two-Step Autoinjectors”. ONdrugDelivery, Issue 152 (Oct 2023), pp 38–42.
Alex Fong discusses the results of a study assessing patients’ ability to switch from a three-step autoinjector to a two-step device.
Biological medicines are currently the largest cost in the medicines budget of the UK NHS, and the largest area of cost growth.1 By making more biosimilar alternatives available, the service expects to save up to £300 million each year,2 while also enabling wider patient access to life-saving and life-enhancing treatments. In the US, one study estimates savings of US$38.4 billion (£30.5 billion) from 2021 to 2025, compared with 2020, as the wider availability of biosimilar products creates a significantly more competitive market.3
With an increasing number of patents for biological medicines now expiring, we are closer to seeing whether these estimates are accurate. Adalimumab, initially only available under the brand name Humira® (Abbvie, US), was the world’s top grossing drug prior to covid-19 vaccines.4 Humira’s patent expired in Europe in 2018. In the UK, there are now five adalimumab biosimilars available, under different brand names.5 By enabling access to adalimumab biosimilars, the NHS saved £400 million in the three years following expiry – equivalent to almost a full year’s supply of Humira.6
In the US, Humira lost market exclusivity more recently, in January 2023. The first biosimilar launched in the same month, and a total of eight have now been approved.7 Only one of these, Cyltezo® (Boehringer Ingelheim, Germany), has interchangeability status, although others have applied to the US FDA for designation.8 As more biosimilars enter an increasingly competitive market, more companies are aiming for interchangeability status to gain commercial advantage.
PROTECTING THE PATIENT EXPERIENCE
Cost is not the only consideration when switching from biologics to biosimilars; the patient experience may also be affected by any change in drug formulation or the drug delivery device provided. As one study notes, “there is scarce information on the patient’s attitude toward such switching, especially studies comparing the injection devices”.9 To identify the most suitable device for their biosimilar product safely, pharmaceutical companies should have access to data from human factors testing and other data attesting to device ease of use. This data would then also support regulatory applications for interchangeability determination.10
In the US, an interchangeable biosimilar drug may be substituted at the pharmacy for the reference product without the intervention of the prescriber. Not all biosimilars, however, have interchangeable status. Companies must apply to the FDA for their product to be approved as an interchangeable biosimilar, providing adequate information to support an interchangeability determination.
In contrast, biosimilar medicines approved in the EU are already deemed interchangeable with their reference medicine or with an equivalent biosimilar.11 The EMA specifies that, “Interchangeability in this context means that the reference medicine can be replaced by a biosimilar without a patient experiencing any changes in the clinical effect”.11 Unlike in the US, “Any decision on switching should involve the prescriber in consultation with the patient”.12 However, individual member states manage decisions regarding “substitution” at pharmacy level, where substitution means to dispense one medicine instead of another without consulting the prescriber.13
When it comes to the device component, the FDA stipulates that any regulatory application should provide evidence that the impact of switching between delivery devices has been assessed, stating, “Data and information supporting the appropriate use and performance testing of the delivery device constituent part of the proposed interchangeable product should be submitted.”14 For manufacturers of biosimilar products, it is therefore important to de-couple the device element from any biosimilar interchangeability clinical study, to ensure thorough risk assessment.
In Europe, guidance from the EMA separates the drug from the delivery device, allowing for differences in the administration device, as long as there is no impact on safety and efficacy. Clinical switch studies for biosimilar drugs are not required in the EU, but prescribers and policymakers may assume that they are necessary and hesitate to make decisions without clinical data. This may be reflected in attitudes to device switching, especially as there is a lack of extensive guidance and existing evidence on this aspect of interchangeability.
“The study aimed to determine whether regular users of a market-leading three-step autoinjector can switch to a two-step autoinjector and perform the injections successfully at first and second time of use.”
A DEVICE SWITCHING STUDY
Recognising the need for a greater evidence base and to support pharmaceutical companies in de-risking their choice of device, Owen Mumford commissioned an independent study assessing patients’ ability to switch between two different autoinjectors. Biologics suitable for subcutaneous administration are now routinely delivered using autoinjector devices, in large part because they offer convenience and allow patients to self administer medication in their own homes without a healthcare professional. The study aimed to determine whether regular users of a market-leading three-step autoinjector can switch to a two-step autoinjector and perform the injections successfully at first and second time of use.
The three-step autoinjector used in the study was SHL Medical‘s DAI®, a button-activated device that was one of the first modern autoinjectors to be commercialised for home injection.15 Study participants switched between the DAI and Aidaptus®, a two-step autoinjector manufactured by Owen Mumford Pharmaceutical Services.16 With two-step devices such as Aidaptus, it is not necessary to push a button as injection is activated through pressure, i.e. when the device is pressed onto the injection site. The key features of the two autoinjectors are summarised in Table 1.
| General Information | DAI® | Aidaptus® |
| Device Type | Single-Use Disposable Autoinjector |
Single-Use Disposable Autoinjector |
| Activation Type | Combined Pressure and Button |
Pressure |
| Needle Insertion | Automatic | Automatic |
| Needle Removal | Manual | Manual |
| Geometry | ||
| Total Device Length (pre use) | 153 mm | 162 mm |
| Evidence Device Diameter | 18 mm | 18 mm |
| Weight (including fill volume) | 34.4 g | 35.3 g |
| Activation | ||
| Activation Force | 8.6 N (button) | 15 N (shroud) |
| User Hold Force | 2.8 N | 4 N |
| End Of Dose Indication | ||
| Visible | Yes | Yes |
| Audible | Yes | Yes |
| Tactile | No | No |
| Dication | ||
| Delivered time | 1.8 s | 1.8 s |
| Delivered dose* | 0.49 mL | 0.49 mL |
| Exposed needle length | 5.3 mm | 5.3 mm |
Table 1: Technical data comparison between DAI and Aidaptus.
STUDY PARTICIPANTS
An independent research agency conducted the study. A total of 52 tests were conducted, with 26 participants in the UK and 26 participants in the US. All participants had been using the DAI device for at least three months. Figure 1 shows the breakdown of participants by gender and age. The average age was 51 years. In the research conducted in September and October 2021 in the UK, 18 women and eight men, aged 16–65 years, participated. In the research conducted in April 2022 in the US, 16 women and 10 men, aged 41–75 years, participated.
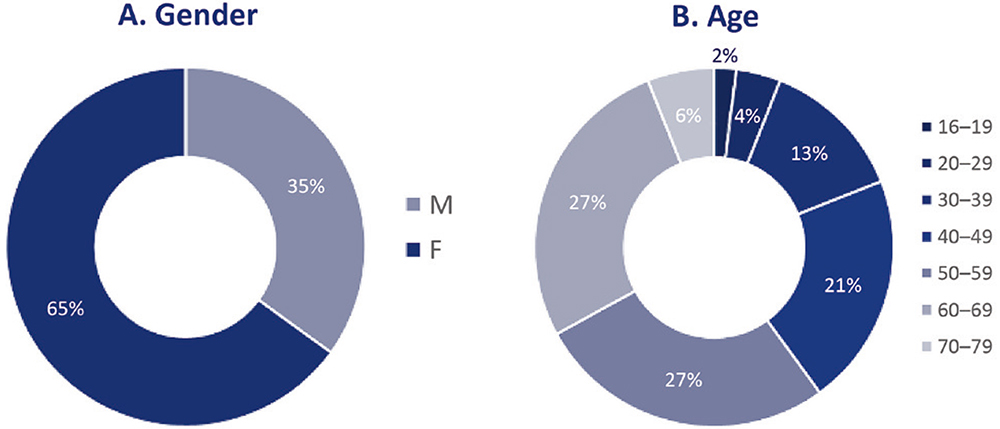
Figure 1: Breakdown of participants based on (A) gender and (B) age.
METHODOLOGY
The study followed the FDA guidance document published in May 2019: “Considerations in demonstrating interchangeability with a reference product”.17 Participants were asked to complete four injections, alternating between DAI and Aidaptus autoinjectors (Figure 2). This study was not intended to be a direct comparison between the DAI and Aidaptus autoinjectors, as all participants were already familiar with the DAI. Rather, it tested participants’ ability to learn how to use the new Aidaptus autoinjector, to switch from a familiar to a new device, and to successfully complete injections with a new autoinjector.
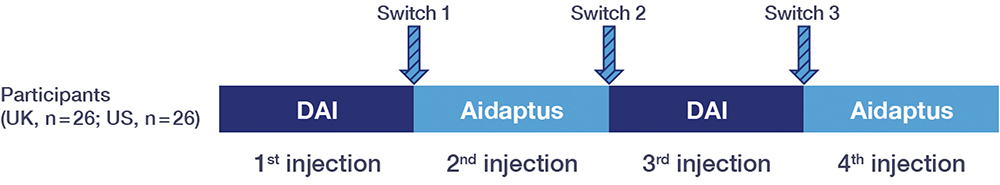
Figure 2: Study design – alternating autoinjection device.
Each participant was provided with the following:
- Two Aidaptus devices in individual boxes (i.e. one device per box) containing instructions for use (IFU): 0.5 mL sterile water for injection in a prefilled syringe
- Two DAI provided in individual boxes (i.e. one device per box) containing IFU: 0.5 mL drug volume in a prefilled syringe
- One injection pad
- Two sharps bins (one on the table for the devices, one on the floor for the injection pad)
- Gloves (the option of wearing gloves was given due to covid-19 concerns).
“Ease of use was calculated by watching how participants handled and examined the device, as well as the time taken for injections.”
This was the first time participants had been presented with Aidaptus and they did not receive training, a demonstration or coaching. Participants were provided with the devices in unopened original boxes (containing their respective IFUs) and asked to administer injections into an injection pad placed on a table. Facilitators were briefed to intervene only in instances where there was a risk to the participant.
The primary measure was injection success. Injections were considered successful if the participant correctly delivered the injection into the pad, as described by the IFU, and allowed the contents of the autoinjector to be fully delivered into the injection pad before removing it. Aside from injection success, other measures were calculated by analysing videos of participants throughout the test. Ease of use was calculated by watching how participants handled and examined the device, as well as the time taken for injections. To calculate time taken, an injection was considered to have begun once the participant placed the injector on the injection pad and initiated the injection process, and to have ended after the participant removed the injection device from the injection pad.
RESULTS: EASE OF SWITCHING
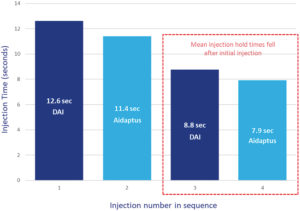
Figure 3: Mean time taken for each injection with DAI and Aidaptus (n = 52).
The user tests had a 100% success rate for both devices, meaning that each injection in the sequence was delivered and completed successfully.
Injection Time
Although participants were familiar with the three-step autoinjector, DAI, they took more time for the initial injection than with the two-step autoinjector (Figure 3). On average, the first Aidaptus injection times were 1.2 seconds faster than the first DAI injection times. For the second injection, mean times of both the three-step (DAI) and the two-step (Aidaptus) autoinjectors were similar, at 8.8 and 7.9 seconds, respectively.
Further Observations
- Geography: US participants were, on average, 4.6 seconds faster than UK participants in delivering the first Aidaptus injections; however, times were similar for the second injections.
- Sex: the average time taken for injections for women across Aidaptus injections was 30% longer than for men (Figure 4). There was only a very small difference (one second) between the total time taken across both DAI injections.
- Handedness: for left-handed participants, the time taken to deliver both Aidaptus injections (27.5 seconds) was similar to the time taken to deliver both DAI injections (28.4 seconds).
- Age: older participants (over 40 years old) took marginally longer to deliver their injections for both devices but still completed injection successfully.
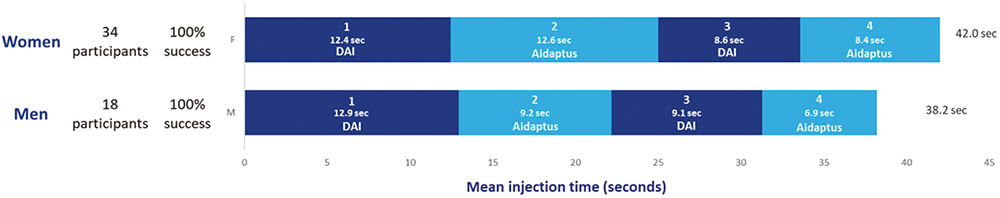
Figure 4: Mean time taken to deliver injections for women and men (n = 52).
“Since participants did not receive training or a product demonstration, but did have access to an IFU, the study observed how they approached the injection process.”
IFU and Device Examination
Since participants did not receive training or a product demonstration, but did have access to an IFU, the study observed how they approached the injection process. A total of 23% of participants were labelled as “cautious”, as they examined the device and/or IFU before every injection. The study environment and the fact they were being observed may explain why participants took the time to examine both the familiar and new device. Across all injections, cautious participants spent longer examining the device and delivered their second injections more quickly than their first injections. Meanwhile, “impulsive” participants (8%) delivered their second injections faster than non-impulsive participants and in the same average time across the two devices. Impulsive participants were those who never examined the device or IFU across all four injections.
Further Observations
- Ease of use: most participants needed to examine the device only once to deliver injections successfully.
- Geography: a higher percentage of US participants (92%) than UK participants (85%) examined the IFU and device before their first Aidaptus injection. Before the second Aidaptus injection, 73% of UK participants examined neither the device nor IFU, compared with 54% in the US.
- Sex: for the first injections using the DAI and Aidaptus, more men examined the IFU and/or device than women. Before the second injections, a greater proportion of women examined the IFU and/or device than men. However, gender did not impact the success of injections or the ability to switch between the two devices.
- Age: the percentage of participants who looked at the IFU and/or device prior to injecting across all four injections increased with age.
SUMMARY
The results of this study clearly showed that all participants were able to switch successfully from the three-step autoinjector, DAI, to the two-step autoinjector, Aidaptus, with no impact on injection success. Participants used the two devices in asimilar way, with injection times falling after each injection, indicating familiarisation. When first given Aidaptus, most participants examined the device or looked at the IFU (or both) prior to beginning the injection process. After the first injection, nearly all participants were able to use a twostep autoinjector as easily as a three-step autoinjector, with no additional need to refer to the IFU or device to achieve very similar injection times. Mean injection times were similar for both devices. Demographic factors such as age, gender or handedness, and behavioural factors such as impulsiveness or cautiousness did not impact injection success.
CONCLUSION
The choice of injectable drug delivery devices can help pharmaceutical manufacturers to differentiate their combination products, especially as the market becomes increasingly crowded with biosimilars. However, switching of drug delivery devices should maintain (and ideally improve) the patient experience and have a limited impact on patient behaviour.
Multiple factors may be at play in the switching process, and more studies are needed for a thorough understanding of these. One study on this topic assessed patient experiences in a switch from Humira to one of its biosimilars in Iceland.9 It revealed the impact of requiring patients to switch to a biosimilar, of modifying drug formulation, of changing the type of needle and of a lack of training uptake. However, unlike the user test described in this article, the Icelandic study was based on telephone interviews rather than a formalised switching trial according to FDA guidelines. Other similar trials focusing on the patient experience after switching are underway.18,19
The user study discussed in this article focused on the device itself, decoupling the drug delivery device element from the combination product. All of participants switched devices successfully, without external intervention, effectively de-risking the choice of a two-step autoinjector in place of a three-step device.
Find out more about Owen Mumford’s Aidaptus® autoinjector at: www.owenmumford.com/en/aidaptus.
REFERENCES
- “Medicines: improving outcomes and value; Biosimilar medicines”. Web Page, NHS England, accessed Aug 2023.
- “Medicines: improving outcomes and value; Biosimilar medicines”. Web Page, NHS England, accessed Aug 2023.
- Mulcahy A, Buttorff C, Finegold K et al, “Projected US savings from biosimilars, 2021 –2025”. Am J Manag Care, 2022, Vol 28, pp 329–335.
- “Nature reviews drug discovery: biobusiness briefs; Top product forecasts for 2023”. Web Page, Nature, Dec 2022.
- “Medicines A to Z; Adalimumab”. Web Page, NHS England, accessed Aug 2023.
- “NHS saves £1.2bn in drive to switch to cheaper versions of medicines”. Pharm J, Jul 5, 2022.
- “What Do the Humira Biosimilar / Interchangeable Launches Mean for the Adalimumab Market?”. JD Supra, Aug 8, 2023.
- “FDA Accepts BLAs Supporting Interchangeability From Alvotech, Pfizer for Biosimilars to Humira”. AJMC Center for Biosimilars, Feb 28, 2022.
- Karlsdottir K, Gunnarsdottir A, Grondal G et al, “A Patients’ Perspective Towards the Injection Devices for Humira® and Imraldi® in a Nationwide Switching Program”. Front Med, 2022, Vol 9, article 9799494.
- “Biosimilars”. Web Page, US FDA, accessed Aug 2023.
- “Biosimilar medicines can be interchanged”. EMS, Sep 2022.
- “Biosimilars in the EU”. Web Page, EMA, 2019.
- “Biosimilar medicines can be interchanged”. EMA, Sep 2022.
- “Guidance for Industry: Considerations in demonstrating interchangeability with a reference product”. US FDA, May 2019.
- “DAI®”. Web Page, SHL Medical, accessed Aug 2023.
- “Aidaptus®. Company Web Page, Owen Mumford, accessed Aug 2023.
- “Guidance for Industry: Considerations in demonstrating interchangeability with a reference product”. US FDA, May 2019.
- “Yuflyma® (Adalimumab), Patient Experience After Switching”. Web Page, ClinicalTrials.gov, accessed Aug 2023.
- “Observational Study, to Assess Treatment Retention of an Adalimumab Biosimilar (Hyrimoz®) in IBD Patients in Real Life Setting (HYRISS)”. Web Page, ClinicalTrials. gov, accessed Aug 2023.

