Citation: Strothers S, “F2V: a Sterile, Customisable Liquid Fill-Finish Platform for the Total Lifecycle”. ONdrugDelivery Magazine, Issue 101 (Oct 2019), pp 48-54.
Simon Strothers presents an integrated fill-finish platform, F2V, designed to represent a commercial filling system as a bench-top solution. The article also includes a recent case study where the F2V was used by Consort Medical to help develop and industrialise a flagship delivery device.
Current market trends indicate that next-generation biopharmaceuticals will have a major impact on the way drugs are formulated and the devices that deliver them. The pharmaceutical industry is also increasingly seeing a rise in the development of personalised medicines, which are tailor-made solutions designed specifically to meet the needs of a particular patient. These drug products require low-volume manufacture and a high degree of flexibility. Regulatory pressures to improve the patient experience are also driving new combination products and associated device developments. Many companies involved in this area are small start-ups, university spin-offs and venture capital-backed businesses usually with some novel intellectual property.
Production of parenteral products, especially primary drug filling and finishing, demands sterile production environments with aseptic manufacturing systems and know-how. Regulators are demanding that parenteral manufacturers “automate more” during preclinical and early clinical phases to reduce human operator influences on the process to improve product quality and safety.
The combined effect of the above is having a significant impact on existing supply chains and manufacturing models. Equipment innovation and sterile facilities are required that can fill a much wider variety of container formats in smaller volumes. There are new challenges for liquid filling; higher accuracies, highly viscous products and multi-product devices demanding more advanced pump technologies and inspection methods.
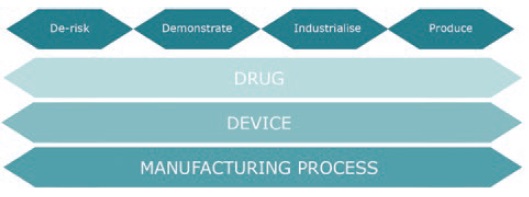
Figure 1: A simple framework to consider when planning and resourcing for a new drug or device development project.
PLANNING FOR SUCCESS
Built on previous project experience we propose a simple framework to consider when planning and resourcing for a new drug or device development project (see Figure 1). The four headings represent the key activities that should be considered when developing a new combination product. Drug, Device and Manufacturing Process are the three main work-streams that typically demand different resources and skills sets and which should be considered in parallel to ensure the interdependencies are explored, defined and considered from the start.
F2V – LIQUID FILL-FINISH PLATFORM
With the objective of helping customers to address all of the key activities and workstreams highlighted in Figure 1, 3P has developed F2V, an isolator-ready, flexible, customisable platform for both filling and finishing of a wide variety of containers and devices including syringes, cartridges, vials, bottles and customised drug containers.

Figure 2: F2V sterile fill/finish, configured for syringe filling with nitrogen purge and vacuum stoppering.
Whereas most lab-scale systems offer individual process stations only, F2V (shown in Figure 2) provides a GMP-compliant, all-in-one integrated system for nitrogen purging, liquid filling, vacuum stoppering and other processes on the same machine. This increased level of automation improves productivity, product quality and patient safety, whilst still providing the flexibility you would expect from a bench-top, low-volume system. Operators are not required to transfer containers between the critical fill and stoppering processes, thereby simplifying the process and reducing risk.
With ongoing support from the 3P team, the F2V platform is fully customisable and comprises a series of fully programmable, servo-controlled modules for bottom-up filling, nitrogen purging, vacuum stoppering and stopper pick-and-place. A separate bench-top Rotary Crimper is also available and enables customers to complete the finishing process for cartridges and vials. An easy-to-use, touch-screen display enables easy adjustment of speeds and strokes to suit individual container types and dimensions.
Recipes can be created and recorded to suit different container types and sizes. Once recorded, the associated parameters will be stored against the named recipe. Operators can then reselect a recipe at a later date to retrieve the exact same settings and conditions for production. This option speeds up changeovers between batches and supports development activities such as design of experiment (DoE) and sensitivity analysis.
Containers are loaded manually into a transfer puck. Pucks are custom-designed, specific to a particular container type. The puck is connected by to a servo motor-controlled arm which gently moves the container between the filling and finishing stations. A gentle motion profile prevents spillage or unwanted movement of liquid up the sides of the container, which may affect stoppering and the sterile barrier.
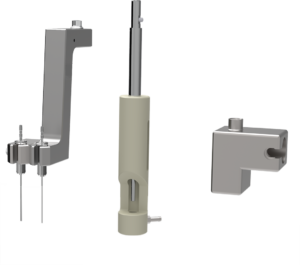
Figure 3: F2V change tooling set can be rapidly changed over to take different container sizes and types.
The transfer puck forms part of the F2V change tooling set (Figure 3) and can be rapidly changed over to take different container sizes and types. For example, following filling of a batch of cartridges, F2V can be quickly changed over to fill a batch of vials. As required, this could be with a different pump technology, a different liquid and a different dose weight.
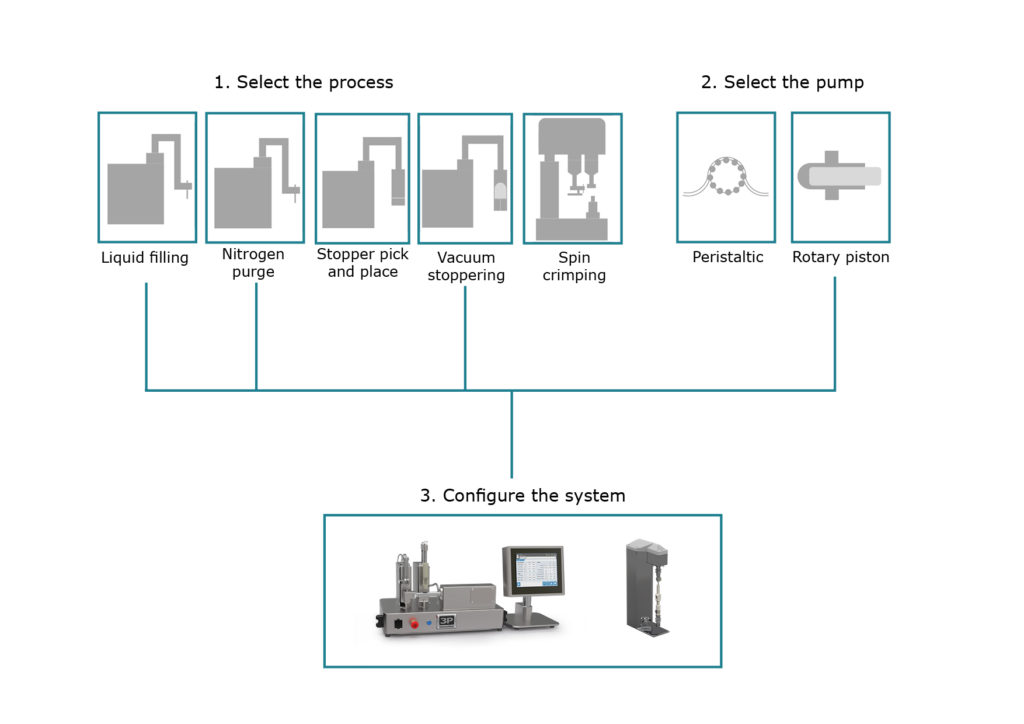
Figure 4: A new system is configured by choosing the required processes and pump type.
Complete Lifecycle Service
3P provides a complete lifecycle service to support its machines in operation. During the container development phase, new change parts can be 3D-printed by our engineers to enable initial test fills and supply of samples. As a design becomes firm, 3P will support with manufacture and supply of GMP-compliant change parts, suitable for clinical and production manufacturing.
A major benefit of F2V is therefore its ability to accommodate late-stage changes in device and container design. F2V is intentionally designed as a flexible and versatile production platform rather than a single, fixed machine. An F2V system can be configured to suit specific processes which may be applicable to individual containers or a range of container types. Figure 4 shows how a new system is configured by choosing the required processes combined with a choice of pump type.
It is also possible to supply multiple pump types for a given process. For example, we can supply rotary piston pumps, more suitable for higher-accuracy or higher-viscosity drug products as well as peristaltic pumps, ideal for single-use, biopharmaceutical filling.
F2V STATIONS AND THEIR APPLICATIONS
Different F2V stations perform specific functions supporting the development and manufacture of various container types (see Figure 5).
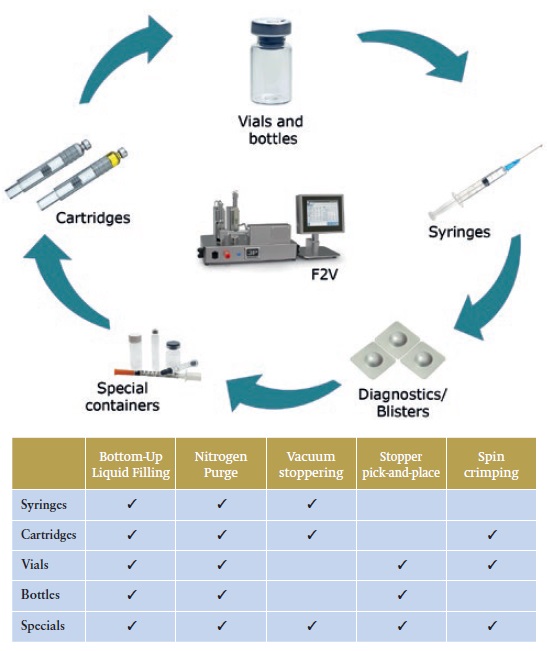
Figure 5: The functions and container types that different F2V stations support.
Liquid Filling and Nitrogen Purge
The liquid filling module (Figure 6a) integrates with any pump format as required to suit the application, including peristaltic, and rotary piston pumps. A wide choice of nozzle types and sizes is available to suit the dose volume, liquid and container type. Programmable bottom-up filling enables fine adjustment of needle position, speed of lift and fill rate, avoiding splashing, frothing or contamination of the stoppering zone. A nitrogen purge option is available which uses the same programmable controls as for liquid filling.
Pump Innovation
The novel rotary pump module (Figure 6b) from 3P’s pump partner, Bio Solutions Gate (BSG, Francheville, France), compliments the F2V system, providing the same degree of flexibility and reconfiguration in one integrated system. Isolator-ready, the compact, bench-top system uses high-quality ceramic pumps from Neoceram (Strépy-Bracquegnies, Belgium), providing very high accuracy and repeatability, ultra-low particle release, no interaction with the drug product and long life-time capability. Connected to the F2V system, the BSG pump can fill liquids from 0.03-175 mL (depending on selected ceramic pump) with an accuracy up to ±0.1%. Toolless assembly is enabled by BSG’s fast-locking concept. An alternative dosing unit is also available, dedicated to micro-dosing and capable of fill volumes from 0.01-8.5 mL, also with an accuracy up to ±0.1%.
Vacuum Stoppering
Suitable for closing cartridges, syringes and other special containers, the vacuum stoppering station (Figure 6c) integrates with a standard vacuum pump. Vacuum levels can easily be adjusted and a sensor ensures target vacuum has been achieved prior to motorised insertion of the stopper. The stopper insertion depth is finely adjustable via the touch-screen human-machine interface (HMI).
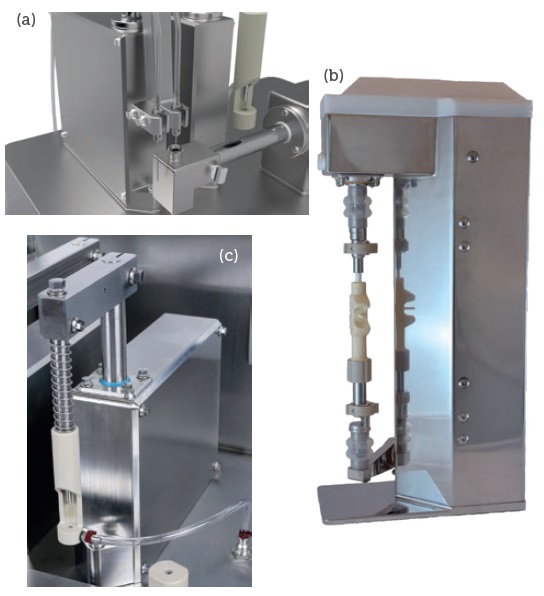
Figure 6: Liquid filling module (a), isolator ready rotary pump (b), vacuum stoppering station (c).
Stoppers are loaded manually into a device-specific housing. This housing forms part of the F2V change tooling set. All change tooling can be removed quickly and easily, without the need for tools or equipment and can be disassembled, cleaned and sterilised using conventional sterilisation equipment.
Stopper Pick and Place
The stopper pick-and-place station uses vacuum to pick and transfer stoppers and caps to close vials, bottles and other special containers. Stoppers and caps are loaded manually, followed by automated picking and placement onto the container.
Scale Up and Scale Out
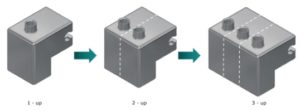
Figure 7: Scale up and out – container puck can be designed to hold one, two or three containers at a time.
The F2V platform is designed to enable fast, easy scale-up through simple change parts and, if necessary, adding additional pumps to the system. Figure 7 shows how the container puck can be designed to hold one, two or three containers at a time. Filling needles and change tooling for the vacuum stoppering station are similarly configured to process one, two or three products at a time.
When higher volumes are required, 3P supports with the design and supply of faster machines or, as indicated previously, through scale-out by implementing multiple F2V machines, which may be sufficient to generate significant volumes for late-stage clinical and even commercial volumes depending on the product and the market demand.
F2V – Rotary Crimper
The spin crimper is supplied as a separate module to enable segregation from the filling and finishing processes. This is typical best practice to minimise risk due to generation of particulates. The module is designed for aseptic processing, suitable for use in sterile isolators and for fully automated and manual sterilisation processes such as hydrogen peroxide vapour (HPV) sterilisation.
A CASE STUDY – LAB-SCALE LIQUID FILLING USING F2V
The following case study provides an example application of F2V for a drug delivery device client. Although it focuses on a nasal device, the equipment used, processes developed, lessons learnt and feedback from the client, Consort Medical’s Aesica Pharmaceuticals division (Queenborough, UK), are all directly relevant to parenteral delivery device filling applications where there are numerous crossovers and similarities.

Consort Medical has developed the innovative Unidose™ Xtra nasal spray device (Figure 8) to provide unique performance advantages through a novel, patented device actuation and drug delivery solution. The device was discussed in detail in an interview with ONdrugDelivery earlier this year. Unidose™ Xtra is compact, simple and intuitive to use, with a low actuation force and a reliable drug delivery that is independent of the force or speed being provided by the end user.
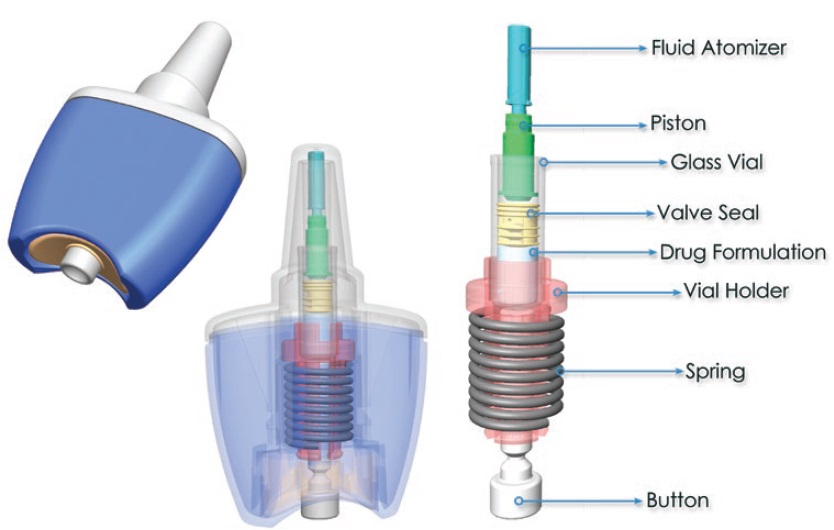
Figure 8: Consort Medical’s single unit-dose, spring powered nasal delivery device, Unidose™ Xtra.
Consort Medical provides its pharma partners with API and finished dosage formulation development and manufacturing service for the device, and as part of this service had a requirement to establish a highly flexible but scalable liquid filling and stoppering system capable of precisely filling a wide range of different liquids and viscosities. In addition, the system needed to be designed for GMP manufacture and capable of nitrogen flushing the drug container for oxygen-free filling and stoppering of their cartridge.
In response to the above, and conscious of the increasing pressures from regulatory bodies such as the US FDA to automate more to improve the safety of drug manufacturing for both end product and operators, Consort selected 3P’s F2V as a versatile, lab-scale liquid filling and vacuum stoppering system.
The core filling and stoppering processes are automated and inherently scalable, avoiding the need to revalidate the process when faster production speeds and higher throughput is required.
The machine has helped Consort Medical by providing:
- A cost-effective means to develop and derisk new devices and combination products at an early stage in their project.
- Valuable insight and clear understanding of manufacturing process and capability – providing data essential to ensure a robust manufacturing process that will stand up to scrutiny and validation.
- Top quality equipment with which to demonstrate manufacturing know-how and scale-up route to their end customers.
- The means for quick and easy manufacture of preclinical and GMP volumes of product to support clinical trials, performance testing, human factors studies, stability trials etc.
CONCLUSION
In addition to the equipment it supplies to its partners, 3P’s reputation is built on providing first-class service and support. The combination of these elements – equipment with service and support – is one of the key factors for project success. Box 1 provides a summary of other key factors to include, together with pitfalls to avoid, to ensure successful project outcomes. Developing and refining device design has been made easier by F2V. This invention allows for the end-product to be tested sooner to measure feasibility quicker. In addition, the individual unit operations are representative of production, meaning that validation does not need to be repeated when a scaled-up solution is required. The F2V is flexible, scalable, easy to use and clean, and can be integrated with other modules for higher-volume semi-automated and fully automated production. The result is a faster product launch and faster return on investment.
BOX 1: KEY FACTORS FOR PROJECT SUCCESS
The following paragraphs summarise some of the more common issues and pitfalls that 3P has seen occur across many projects and over several years, and – importantly – how to avoid them.
Establish a Multi-Functional Team
Running a “skewed” project team is a common pitfall as it is difficult to form a team that contains the right breadth and depth of skills needed to deliver a whole project. For example, a company’s main focus is on device-drug functionality and efficacy but the aspiration is to develop and industrialise a finished solution, including the drug product. Typically, limited time and focus will be afforded to the manufacturing process, how the device is filled, how it might vary in production and how the device impacts the manufacturing process. The result will be a limited understanding of the variables that can affect both functionality and manufacturing processes. This means that technical risks will remain unaddressed – ultimately resulting in significant project delays, set-backs or even cancellations depending on the nature of the issue.
The solution sounds obvious but is often ignored. Establishing a multi-functional team and bringing in the right expertise at the start will raise the correct questions at the start and will drive a more balanced programme which addresses both drug, device and their interdependencies in parallel.
Specialist life science development and automation companies such as 3P augment project teams, helping to generate a holistic view of the drug, device and manufacturing process interactions leading to a smoother commercialisation/industrialisation path.
Keep the End Goal in Sight
Closely related to the skewed project team issue, projects often suffer from a short-term view, often as a result of the wrong scope of objectives being defined at the start. For example, a device design and development team might focus on achieving key functionality in the device, but without considering how it will be filled or tested. The result is that design changes are often required to enable filling or manufacturing at commercial scale, causing delay and significant on-costs.
The solution is the same as above – a multi-functional team, formed at the earliest stages and involved in project planning and objective setting, as well as in the design and development process from the start, will ensure that all essential aspects of the project are considered at the right time to avoid rework.
Build a Complete Process Understanding
Process understanding is a key aspect in device development. In essence, it involves applying physics and quantifying the range of conditions under which a component (e.g. a liquid, a powder, a plastic component, etc) will behave in the desired way for the product to give the required function. The engineering skills involved are often different to the skills involved in generating new design concepts.
Successful teams will pull in the right mix of resources to ensure science is applied to build a full understanding of every aspect of a new product, including all pertinent processes. Well-proven and tested tools such as failure mode effects and criticality analysis (FMECA), design of experiment (DoE), sensitivity analysis, design for manufacture/design for assembly (DfM/DfA) are invaluable to test design robustness for both function and manufacturability.
Automation and engineering experts such as 3P fulfill a key consulting role in providing support to customers across all of these areas, resulting in an optimised product as well as process for manufacture.
Consider Early How to Manufacture
Don’t wait too late to consider how to manufacture a device. An all too common scenario unfolds when a company has completed the drug and/or device design and development work and is now ready to manufacture initial volumes. This is a critical stage for any project and is often where many finish up cancelled or postponed. The reason for the failure is so often due to lack of attention and resources dedicated earlier on to exploring manufacturing solutions and/or unrealistic estimates of how much a manufacturing solution will cost and how long it will take.
To avoid these issues, engage with equipment suppliers at the earliest stages of the project to provide drug and device development projects with the crucial information required to make the decisions that can influence design and enable a faster, smoother route into clinical manufacture and beyond.
Be Ready to Demonstrate Manufacturing, Know-How to Your End Customers
Having a great device or a great drug product in isolation is generally not enough to persuade big pharma to invest and take it forward to commercialisation. As end customers, big pharma companies are looking for and expect the complete package, where drug, device and manufacturing solutions are available, tested and proven.
It is not sensible nor financially viable for a device developer to invest at the outset in the high-speed, fully automated production lines which would ultimately be required. However, what is possible is to show end-customers that the key high-risk production processes have been defined, developed and derisked.
The way to achieve this is by investing in low-volume, bench-top machines. These can be GMP compliant and fully representative of individual process steps, but will typically require operators to complete the lower-risk steps, such as container load/unload and transfer between processes. Portable and versatile, machines can easily be installed in clean rooms and isolators.
Learn to Walk Before You Run
Aiming for a single, fully automated, high-speed machine that will meet volume targets projected for 3-5 years’ time is not always the most sensible approach. Depending on the level of commercial risk that exists on the project, such as untested or unproven market demand, businesses may be better off scaling out, rather than scaling up, to deliver increasing volumes.
Having already provided manufacturing capability through bench-top machines, further volumes can easily be achieved by procuring multiples of the same machine. Obviously, more operators will be required, but this is often faster, lower cost and carries less risk than a high-speed, fully-automated solution.
The critical point to understand here is that although the product unit cost may be higher, the ability to match output with growth in demand incrementally using a scale-out approach, rather than by step change (investing in long-lead, high-speed equipment) that comes on stream many years later, means companies can see early growth in their revenue stream.

