To Issue 166
Citation: Hurlstone C, “Future Trends for Novel Injectable Drug Delivery”. ONdrugDelivery, Issue 166 (Oct 2024), pp 14–17.
Chris Hurlstone looks at the increasing demand for novel injectable devices and summarises the areas we can expect to see significant development activities in injectable technologies.
Significant growth is predicted for many areas of the injectable drug delivery sector. Although small-molecule-based formulations dominate pharmaceutical innovator pipelines generally, the development of advanced biologics continues to be a major focus for injectables. Meanwhile others, such as the glucagon-like peptide-1 (GLP-1) blockbusters, are making headlines and impacting supply chains. In terms of therapy areas, many pharma companies are targeting oncology, immunology and cardiovascular fields, with cell and gene therapies becoming increasingly important. Given these predicted advances in drug formulation and other drivers, such as sustainability, where are novel injectable drug delivery devices currently headed?
DRIVERS AND CHALLENGES FOR NEW INJECTION DEVICES
Novel device development is rarely a company’s first choice for a variety of reasons. In the injectables sector, formulators will often target 1 mL subcutaneous delivery or similar where possible, as this is an area already well served by existing devices that are well understood by manufacturers, regulators and users.
However, there is an ongoing and potentially increasing demand for novel devices, driven by many different factors, including servicing of niche markets, such as emergency use. Much of the activity can be attributed to three particular areas:
- Meeting the needs of at-home users, moving treatment out of the clinic and into the home
- Finding ways to add value and improve usability while meeting the demands of cost and carbon footprint reduction
- Targeted drug delivery, for example, direct to organ/tumour.
MOVING FROM CLINIC TO HOME – THE NEED FOR LARGER-VOLUME AND/OR HIGHER-VISCOSITY INJECTIONS
Healthcare systems are increasingly looking to move injection-based treatments from the clinic, currently often administered via intravenous infusion, to the home. In this endeavour, they may turn to delivery systems such as syringe pumps and ambulatory pumps, which are currently undergoing development either as new devices or through the lifecycle management of existing technologies.
Another solution – for lower costs and improved usability – is to move to a subcutaneous injection, via an autoinjector or on-body delivery system, such as a patch pump. The challenge here is that reformulating large infusion volumes down to something that can be delivered subcutaneously will, in many cases, result in dosages of a volume and or viscosity that exceeds the capability of current devices.
“Biologic drugs often also result in the need for injections either of larger volume or higher viscosity, due to the large-molecule nature of their formulation.”
At the same time, biologic drugs, which currently account for 80–90% of injectable drugs in development, often also result in the need for injections of either larger volume or higher viscosity, due to the large-molecule nature of their formulations.
The combined result is an increasing need for new devices that push the boundaries of what handheld autoinjectors can deliver, from a user, device and primary packaging perspective. Many companies are working in this area, with two examples of device technologies being Ypsomed’s Ypsomate 5.5 and SHL Medical’s Bertha (Figure 1).
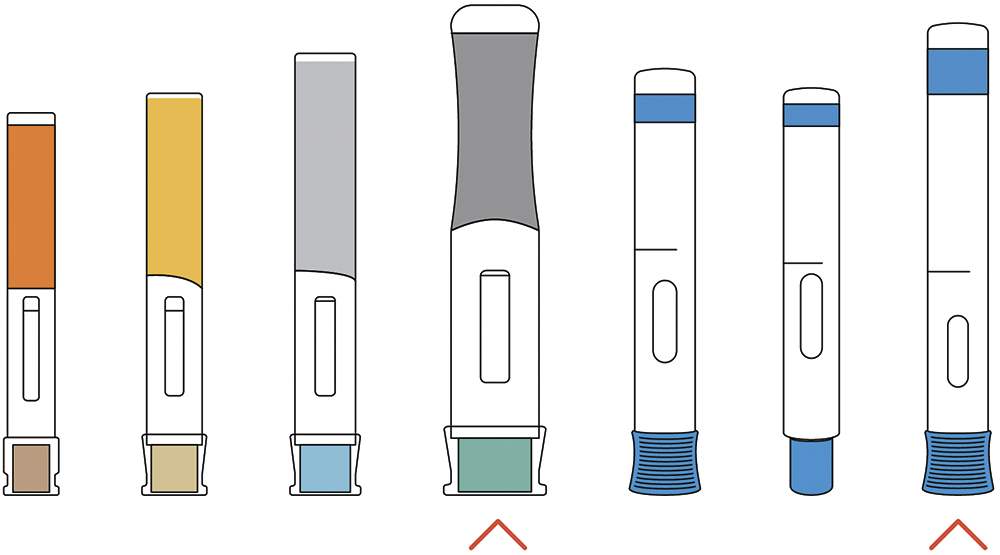
Figure 1: Examples of high-volume and high-viscosity delivery devices.
The Ypsomate 5.5 is available in several configurations and can deliver up to 5.5 mL of drug. The device maintains many aspects of the now industry standard approach, such as overall form and aspect ratio, mode of operation (remove cap, press and hold), inspection window, plus visual and audible indicators. It also uses a prefilled syringe as the primary packaging with a staked needle. However, it is significantly larger than the 2.25 mL device, and a 5.5 mL injection may require an injection time of up to 60 seconds, twice as long as that for the 2.25 mL device. To support the user in achieving this, the device features continuous visual and audible feedback, as opposed to just start and end clicks. The Ypsomed device can also deliver higher viscosities, helping to meet the demand for large-molecule formulation delivery.
Although not able to deliver as high volumes as Ypsomate 5.5, SHL’s Bertha can deliver formulations of 60 cP. The increased forces to achieve this performance, with delivery times of no more than 15 seconds, put more loading on the device and the primary packaging, thus requiring significantly increased mechanical robustness. Although delivery can be achieved in under 15 seconds, the device also features continuous visual and audible feedback to the user.
These systems, among others, are examples of how significant new innovations in device design and engineering are required to extend delivery capability. As performance boundaries and manufacturing methods are pushed to new limits, extra effort is needed to ensure that technologies achieve the necessary levels of robustness and reliability.
There has also been much activity on pump technologies in recent years and several have reached late stages of development. Apart from in the diabetes sector, however, only a few have secured regulatory approval and been made commercially available. However, these device technologies offer opportunities for the delivery of higher-volume and higher viscosity payloads, so will continue to be a focus of much activity and interest. Which succeed and which fall by the wayside will be interesting to see.
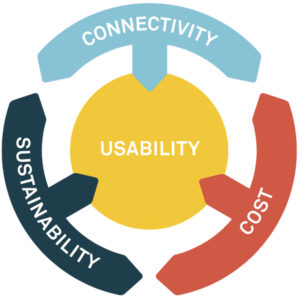
Figure 2: Squaring the current circle of industry trade-offs.
TRADING-OFF COST, SUSTAINABILITY & CONNECTIVITY WHILE MAINTAINING USABILITY
One of the challenges habitually faced by organisations developing new technologies to meet increasing demands is how to find the best trade-off between key constraints. Recent emphasis on the potential value of digital tools and device connectivity, set against the increasing focus on sustainable technology solutions, have resulted in a new spin on this trade-off assessment (Figure 2).
How can you provide the potential benefits of connected systems to the user, the commercial manufacturer and healthcare systems in general, while also meeting potentially conflicting demands of cost and sustainability, all while ensuring that usability is optimised?
This is one of the key questions facing the industry, with different approaches currently being taken to resolve the complex relationships between these opportunities and challenges (Figure 3). The following are some of the options currently being explored in the industry.
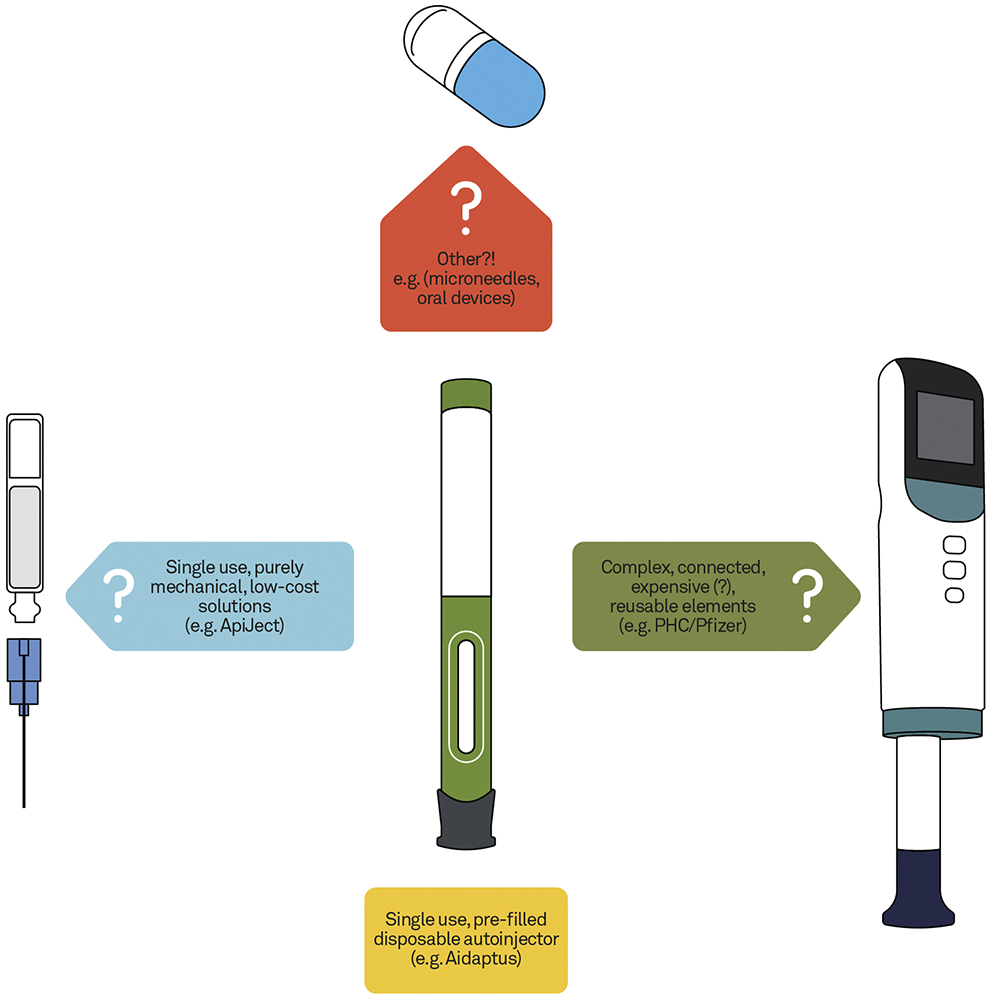
Figure 3: Different approaches to address device trade-offs.
Option 1 – Develop Systems With Reusable/Durable and Disposable Elements
Splitting an injection platform into a “retained”, “reusable” or “durable” element, which incorporates the drive system and much of the caseworks and user interface, can transfer cost and carbon footprint into a separate subassembly that can be used multiple times. For organisations wishing to add value through device connectivity and digital tools, the overheads of achieving this can effectively be amortised across multiple uses.
Challenges include how to retain equivalent ease of use for a system that now requires additional user steps, and how to ensure longer-term reliability of a retained multi-use system. It must also be recognised that quite a significant proportion of cost and carbon footprint of such an autoinjector system remains in the disposable primary packaging sub-assembly, so potential savings should not be over-estimated.
Examples of this approach include electro-mechanical platforms from UCB (Ava connect®), Phillips Medisize (Aria), Pfizer/PHC (Smartclic®) and SHL’s very recently announced Elexy™. Purely mechanical takes on this approach, which forego the connectivity aspects, can challenge cost and carbon footprint even more strongly, but with potentially less added value. New examples of these are expected to appear in the near future.
“From a connectivity perspective, challenges over data capture and handling also present a barrier.”
From a connectivity perspective, challenges over data capture and handling also present a barrier. Efforts to resolve these challenges will continue but, in the meantime, opportunities to use other digital tools and approaches are being developed, for example, through the use of smart labelling and packaging, sometimes linking to mobile apps and website support materials. These could be equally well applied to purely mechanical device solutions.
Option 2 – Keep the Vision Simple
Rather than moving to more complex device solutions, an alternative approach is to keep the device vision as simple as possible, rejecting potential value-add through additional functionality to focus on the best solution to a particular constraint.
One example is the ApiJect single-dose injection device (ApiJect Systems) which is based on the use of well-established blow-fill-seal manufacturing technology in a new application. This approach provides clear and significant benefits in terms of cost and carbon footprint, with the acceptance that it will not be able to offer connectivity. The company has also worked to demonstrate usability, for healthcare providers if not for self-administering patients. Challenges could remain in this area, as well as in the demonstration of drug stability (including under high processing temperatures) for a primary packaging that is novel to many applications.
Another example of a “simple” device vision is the Eco-inject® autoinjector (Eco-inject), which stays much closer to the well-established and accepted autoinjector product format but is manufactured from 100% bio-based polymers. To achieve greater sustainability, this approach does not provide connectivity benefits and perhaps relies to some extent on an acceptance of “softer” compromises in performance (feel, robustness) and a dependence on an as yet unproven material supply.
“Radically different approaches to established autoinjector technologies are numerous, with some more established than others.”
Option 3 – Something Completely Different
Radically different approaches to standard autoinjector technologies are numerous, with some better established than others. One example is the use of micro-needle arrays and patches, the technology and manufacture of which are quite well proven but for which payload limitations may be a major constraint. Alternatively, more novel approaches, such as the oral delivery platforms being developed by companies like Biora (CA, US) and Lyndra Therapeutics (MA, US), could offer significant cost and sustainability savings, if proven to be effective and suitable for industrialisation and commercialisation.
The current prediction is that the more radical solutions mentioned here, which offer real advantages in terms of cost and carbon footprint, are less likely to succeed. This is because of some technical challenges that need to be overcome, as well as the risk-averse nature of the industry. However, if there was a strong increase in regulatory pressure to reduce the environmental impact of drug delivery devices, this could shift the balance of these trade-offs.
INNOVATIONS IN TARGETED DRUG DELIVERY – ONCOLOGY AND CELL & GENE THERAPIES
A significant new area of device innovation, which is being largely driven by developments in both oncology and cell and gene therapies, is that of drug delivery direct to physical target sites in the body, such as organs, tissues and tumours. The need for this innovation is due to several key factors.
Firstly, many target sites are difficult to get to, both in terms of physical access but also due to the need for accurate navigation and positioning in highly variable and personalised environments. Surgical robots can sometimes be used for such delivery techniques but, for various reasons, the best opportunity for innovation may be in standalone delivery devices that can be deployed more broadly and flexibly, for example, through the use of standard laparoscopic methods. Various types of guidance systems that make use of a range of imaging and navigation tools are also often required.
Another reason why one-size-fits-all device solutions are unlikely is the huge variation in payload that such systems often need to deliver, both in terms of dose volumes – which can range from 1–2 mL to 200–300 mL, but also the drug’s physical characteristics, such as viscosity, single/ multi-phase, sensitivity (e.g. to temperature, to shear) and stability.
Controlling distribution and retention within the target site is also critical to ensure that the necessary amount of drug is delivered but also, in some cases, to ensure that neighbouring non-target tissue is not at risk of damage or contamination. Given the huge range of tumour and organ types, this again points to a need for bespoke solutions, alongside the need to fully understand tissue characteristics. This can be very challenging and is best approached through a combination of experimental and analytical methods.
One specific area of interest and opportunity is that of ocular delivery, for example, for gene therapy through the implantation of drugs and drug delivery devices. Although well established as a delivery route, new devices and procedures for intravitreal, subretinal and suprachoroidal delivery are a focus for many organisations. The use of microneedles, including positioning and depth, is critical to deliver to the targeted retinal or subretinal layer.
These and other challenges, such as the need to ensure that delivery technology can be deployed across a wide range of varying healthcare settings, mean that it is critical to begin device developments very early, alongside the development of the formulation. This is frequently the case but not always appreciated.
WHERE INJECTABLE DRUG DELIVERY IS HEADED
This article has summarised a number of different areas where the industry can expect to see significant development activities in injectable technologies over the coming months and years. While the industry has a reputation for being slow moving and risk averse, frequently for understandable reasons – such as the need to ensure patient safety and conform to regulatory requirements – the opportunities for success are clearly there for organisations willing and able to move quickly and decisively.
Previous article
THE PERFORMANCE OF EUROJECT – A BFS-BASED INJECTION DEVICENext article
COMPANY SHOWCASE: PCI PHARMA SERVICES
