To Issue 168
Citation: Simpson I, “High-Dose Drug Delivery – How Far can Autoinjectors Go?” ONdrugDelivery, Issue 168 (Jan 2025), pp 90–94.
Iain Simpson reviews the development of autoinjectors for self-injection and explains that, despite significant emerging trends for biologics, new developments in formulation and autoinjector technology may enable them to continue to be the dominant delivery device for these medications.
INTRODUCTION
Autoinjectors have been on the market for the injectable delivery of therapeutics for chronic diseases since the 1990s; however, the market has grown more rapidly over the past 10 years, driven both by the introduction of new innovative biologics and the launch of biosimilars. To date, as shown in Figure 1, nearly 100 combination products that include an autoinjector have been approved, of which around 80% are mechanical disposable devices, with the rest of the market split between reusable mechanical and electromechanical autoinjectors. More specifically, spring-powered devices, with manual needle insertion and removal, shield-triggered activation and passive needle protection have become the preferred disposable design.
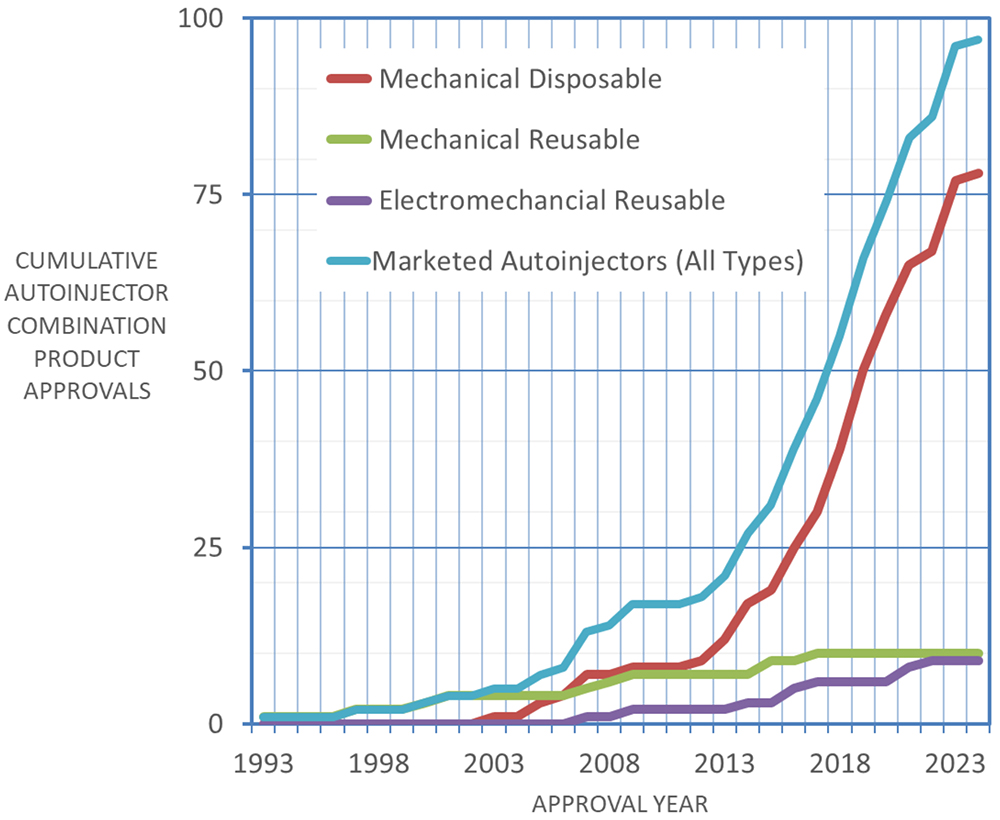
Figure 1: Cumulative autoinjector approvals by year (Data from PharmaCircle, Aug 2024).
Commercially available mechanical disposable autoinjector platforms currently dominate the market, having been approved for multiple combination products and offering economies of scale and confidence regarding development timelines and reduction of regulatory risk. Presently, standardisation is being favoured over differentiation so, even when other device designs offer potential benefits (such as automated needle insertion and retraction to improve comfort or the ability to provide higher injection forces to reduce injection time), trade-offs around cost, size, complexity, development time and regulatory risk need to be considered, meaning they may only enter the market for niche applications.
EMERGING TRENDS IN INJECTABLE DELIVERY DEVICES
Although the trend towards mechanical disposable device platforms looks set to continue, several emerging trends may start to challenge the status quo and disrupt the market.
Environmental Sustainability
Back in 2006, when the first disposable devices entered the market, sustainability was not a major area of concern for the pharmaceutical industry. The benefits of increased self-administration of medication and a focus on ease of use for patients outweighed the negative impact of single-use mechanical devices in terms of cost per dose and sustainability. The same view prevailed in the insulin pen market. However, today, many leading pharmaceutical companies have comprehensive sustainability policies and goals, some of which highlight the need to reduce waste from medical packaging, including delivery devices. As such, more sustainable device solutions are becoming of increasing interest to pharma companies, particularly where large patient populations are involved.
“By adding connectivity to drug delivery devices, real-time medication use data can be reliably captured and used to gather information on adherence and drive digital services that can dynamically adapt to changing use patterns, providing patients with personalised support and feedback.”
Connectivity
By adding connectivity to drug delivery devices, real-time medication use data can be reliably captured and used to gather information on adherence and drive digital services that can dynamically adapt to changing use patterns, providing patients with personalised support and feedback. Although adding the electronics needed for connectivity can add to the environmental footprint of the device, the ability to provide timely support and coaching can potentially reduce disease complications and hospitalisations, which can help reduce the total environmental impact of the treatment, as well as improve healthcare outcomes.
Applying Device Technology Across Multiple Drug Products
Although current mechanical autoinjector platforms are being used across broad drug portfolios, this requires the drug formulation to be confined to a limited range of volumes and viscosities, or for the device to be customised for different fill volumes. While some of the newer mechanical autoinjectors under development can deliver higher volumes and others can be more easily adapted to different primary containers and fill volumes, there are still limitations to their flexibility, but current designs have limitations that might drive the need to look for new technical solutions.
TECHNOLOGY OPTIONS FOR AUTOINJECTORS
One way of categorising autoinjectors is to consider them as being either:
- Single-use or reusable, and
- Having either an electromechanical or non-electromechanical drive mechanism.
The reason for the second classification is that electromechanical devices already incorporate electronics that can enable significant additional functions, such as audible and visual user feedback and inbuilt connectivity, as well as offering benefits for delivery performance. For non-electromechanical devices, electronics could still be added but will not improve the delivery performance of the device. Figure 2 summarises the advantages and disadvantages of the four device options. At present, there are no single use electromechanical autoinjectors as they would have an unacceptably high cost and poor sustainability, although it is worth noting that this approach has been adopted for some on-body devices.
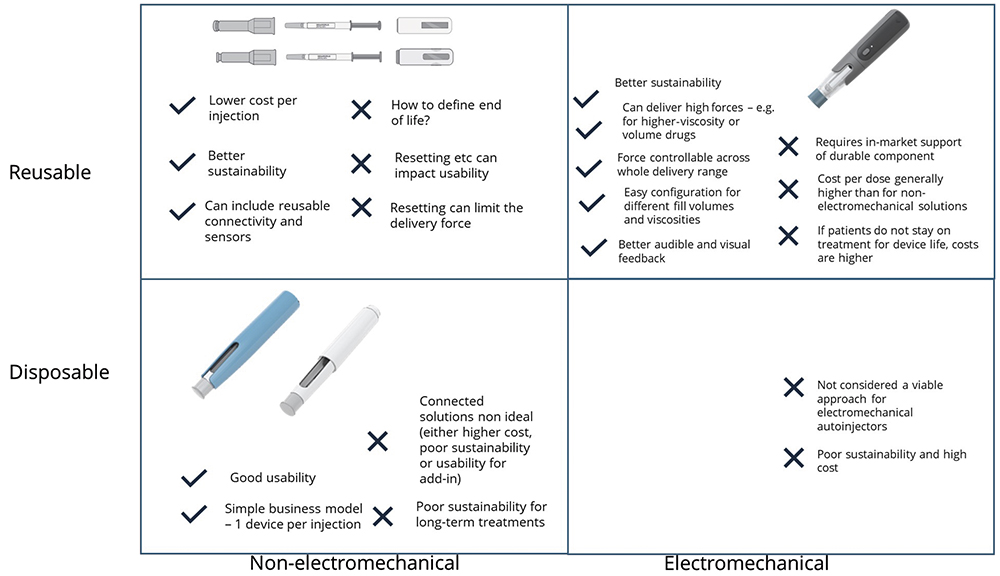
Figure 2: Technology options for autoinjectors.
THE CHALLENGE OF DELIVERING BIOLOGICS – HISTORICAL PERSPECTIVE
In 2008, the prevailing view was that the maximum volume that could be delivered by an autoinjector was 1 mL and the maximum concentration that could be achieved for a stable, injectable monoclonal antibody (mAb) formulation was around 100 mg/mL, suggesting a maximum dose of 100 mg. A drug pipeline analysis from that period suggested that more than 50% of drugs under development would require a higher dose than this, leading to the conclusion that a different type of delivery device would be required to support future self-injection needs if the drug was not to be delivered as an intravenous (IV) infusion.
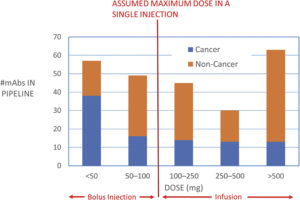
Figure 3: Drug pipeline data from 2008 by dose.
It was suggested that, to facilitate the longer injection times required for these larger doses, wearable on-body devices would be required, and several such devices have been developed or are currently under development to address this need. However, 15 years later, fewer than 10 such drug-device combinations have entered the market and, in contrast to autoinjectors, no dominant design has been established. Figure 3 shows that some of the needs of high-dose delivery anticipated in 2008 have been addressed by increasing drug concentrations up to 150 mg/mL and drug volumes of up to 2 mL.
More recently, there has been increasing interest in moving drugs that are currently delivered as an IV infusion to subcutaneous (SC) injection at a local clinic, or even in patients’ homes. With this trend in mind, a recent systematic review of clinical-stage and approved IV and SC biopharmaceuticals identified 186 biopharmaceutical candidates that would potentially be suitable for large-volume SC delivery with volumes of >2 mL, representing around 15% of all IV and SC biopharmaceuticals.1
It then becomes a question of what the best way is to deliver these doses. On-body devices can deliver a wide range of drug volumes over a variety of delivery times, so could address this need; however, compared with autoinjectors, their usability is less well established and the technology less well proven. To support ease of use, current devices usually have an integrated cannula insertion mechanism and an adhesive means of attaching the device to the body during use. These features mean that reusability is harder to achieve than for an autoinjector, and electromechanical on-body injectors fall into the single-use/electromechanical category shown in Figure 2 where sustainability is poor and cost per use high.
“Arguably, if autoinjectors can deliver higher drug payloads than currently available, they may well compete advantageously with on-body injectors.”
Arguably, if autoinjectors can deliver higher drug payloads than currently available, they may well compete advantageously with on-body injectors. Given the high level of acceptance of autoinjectors in the market today, it makes sense to consider how much further in terms of dose they are able to go, either by considering higher-injection volumes or considering higher-concentration formulations.
OPTIONS FOR HIGHER-DOSE DELIVERY FROM AUTOINJECTORS
Large-Volume SC Drug Delivery
Schneider et al (2023) conducted a systematic review of the literature relating to the high-volume SC bolus injection of therapeutics.2 They highlighted three key considerations relevant to developing large-volume autoinjectors:
- Injection tolerability
- Suitability for self-administration
- Pharmacokinetic equivalence with existing dosing options.
They concluded that high-volume autoinjectors look to be feasible and that further work should be conducted to support their development.
More recently, Calderwood and Lindner presented data at the 2024 PDA Universe of Pre-Filled Syringes conference that showed that large-volume SC injections are clinically feasible and tolerable, higher-capacity mechanical injection systems are technically feasible and that users are capable of handling the longer injection times required to deliver these doses. They concluded that a 5 mL injection from a mechanical autoinjector using conventional drug formulations appears feasible and such a device is under development.
Permeation enhancers offer further options for high-volume injection. Halozyme (San Diego, CA, US) has developed ENHANZE®, a proprietary enzyme that temporarily degrades glycosaminoglycan hyaluronan in the SC layer, allowing a co-administered drug to flow more freely from the injection site, thereby allowing larger drug volumes to be injected successfully without discomfort or risk of drug leakage from the injection site. Using this approach and a new proprietary high-volume autoinjector, Halozyme was able to demonstrate successful injection of 10 mL of representative biologic product co-formulated with its ENHANZE drug delivery technology in 23 healthy volunteers.3 The injection time was around 30 seconds and well tolerated with minimal or mild erythema and swelling that resolved after 90 minutes. All but one of the participants indicated that they would be comfortable receiving the injection again.
High-Concentration SC Drug Delivery
The alternative to delivering higher volumes is increasing the concentration of the drug formulation. A key challenge in doing this, especially for biologics, is that molecular interactions can result in poor stability and the viscosity of the formulation increases rapidly with concentration. However, as Figure 4 shows, this is an area of increasing interest and several companies are actively developing formulation technology to address this need, for example:
- Elektrofi claims that its technology can produce stable and syringeable mAb formulations with concentrations up to 700 mg/mL.4
- Xeris has reported successful formulation and testing of a large molecule at concentrations in excess of 450 mg/mL using its XeriJect technology.5
- Qprotyn (St Petersburg, FL, US) has published results showing reductions in viscosity in a range of mAbs using its HILOPRO formulation compared with the originator at the same concentration.
- Arecor (Chesterford, UK) has developed an aqueous formulation technology, Arestat, that can improve stability and reduce the viscosity of large-molecule formulations.
“Electromechanical autoinjectors could generate larger and more controllable forces than spring-based systems can.”
The aim of these approaches is to develop stable, high-concentration formulations that can be delivered using conventional autoinjector technology; however, ultimately, viscosity and the associated longer injection times will limit what can be achieved using a mechanical drive mechanism. That said, as Phillips Medisize has presented in previous publications,6 electromechanical autoinjectors could generate larger and more controllable forces than spring-based systems can. Figure 5 shows modelled data for a range of drug viscosities and 1 and 2.25 mL injection volumes. The results demonstrate that high-viscosity formulations can be delivered in acceptable times. Furthermore, the superior audible and visual feedback that can be provided by an electronic system may provide better user confidence before and after the injection has taken place.
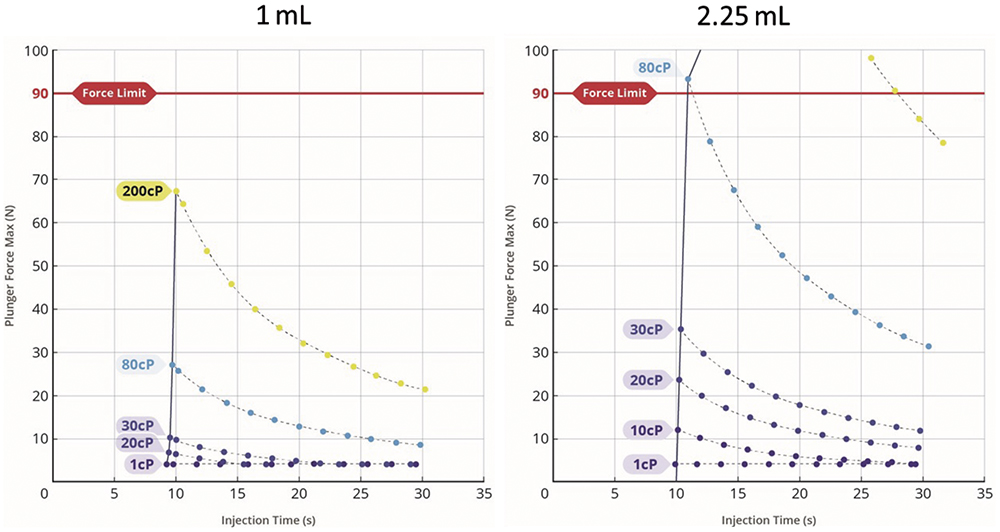
Figure 5: Force-injection time profiles for 1 and 2.25 mL injections. Modelled for an electromechancial autoinjector.
What is the Dose Limit for Autoinjectors?
If work on developing high-volume and high-concentration SC delivery is successful, then dose ranges that can be delivered using autoinjector technology can be significantly increased through a combination of considering high-volume injection, high-concentration formulations and the possibility of two successive injections. Figure 6 shows some possible approaches that could potentially address nearly 90% of the high-dose drug pipeline, offering a credible alternative to moving towards on-body injector devices.
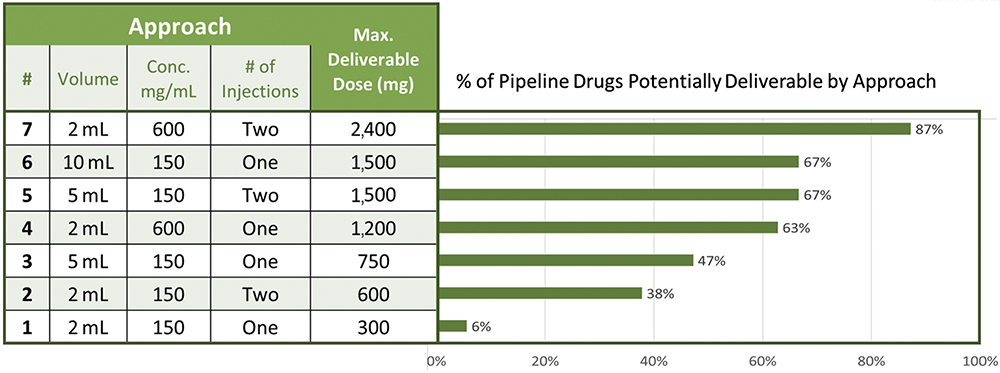
Figure 6: Options for autoinjector delivery of high drug doses.
CONCLUSION
Since 2006, there has been rapid growth in the use of autoinjectors for self-administration of injectable drugs. This approach to drug delivery offers good usability, patient satisfaction and safety. Looking to the future, there is increasing interest in delivering high drug doses by SC injection, rather than IV infusion, with around 15% of the candidate drug pipeline estimated to require injections greater than 2 mL.
It has been argued that this can only be achieved by increasing drug volumes above those that can be delivered by a handheld autoinjector, which has driven the development of on-body devices that can deliver drug volumes of 3–20 mL in a reasonable timeframe (minutes). However, recent developments in autoinjector and formulation technology have the potential to increase the range of doses that autoinjectors can deliver, potentially making them a viable alternative to on-body injectors. Achieving this requires the development of higher-dose autoinjectors and demonstration of their clinical feasibility.
“Compared with mechanical devices, electromechanical autoinjectors can offer superior and more flexible delivery performance.”
Both higher concentrations and higher volumes will require longer injection times to deliver the dose. Compared with mechanical devices, electromechanical autoinjectors can offer superior and more flexible delivery performance. Furthermore, the ability to provide clear audible and visual feedback may increase user confidence and reduce use errors associated the longer injection times. In choosing an appropriate delivery device, several factors need to be considered, including:
- The maturity of technology (device, primary packaging and formulation)
- Usability/user preference
- Cost of goods and sustainability. There is no one size that will fit all, but the strong pedigree of autoinjectors in the market over the past 20 years suggests that they will continue to play a significant role in supporting self-administration of medicines outside a clinical setting.
REFERENCES
- Green P, Schneider A, Lange J, “Navigating large-volume subcutaneous injections of biopharmaceuticals: a systematic review of clinical pipelines and approved products”. mAbs, 2024, Vol 16(1), article 2402713.
- Schneider A et al, “Autoinjectors for large-volume subcutaneous drug delivery: a review of current research and future directions”. Expert Opin Drug Deliv, 2023, Vol 20(6), pp 815–830.
- “Halozyme Announces Positive Clinical Data of its High-Volume Auto-Injector Demonstrating Successful Rapid Subcutaneous Drug Delivery”. Press Release, Halozyme, Oct 2023.
- “Enabling Subcutaneous Delivery of Biologics”. ONdrugDelivery, Issue 97 (May 2019), pp 6–9.
- Prestrelski S, “XeriJect™: Non-Aqueous Formulations for Subcutaneous Injection of Ultra-High Concentration Biologics”. ONdrugDelivery, Issue 133 (May 2022), pp 26–29.
- Sørensen B, “Re-usable Electronic Autoinjector – Flexible Performance”. ONdrugDelivery, Issue 120 (May 2021), pp 44–46.

