To Issue 160
Citation: Bertollo N, Rock A, Byrne R, “How Do You Perceive Intradermal Delivery? Think again…”. ONdrugDelivery, Issue 160 (May 2024), pp 26–30.
Nicky Bertollo, Adam Rock and Ronan Byrne discuss the benefits and limitations of intradermal delivery – and introduce the Pharma Latch Hollow intradermal injection device.
THE POTENTIAL OF INTRADERMAL DRUG DELIVERY
Intradermal (ID) delivery has long been considered an attractive alternative to traditional parenteral delivery strategies for a variety of drug modalities. Compared with other parenteral targets (e.g. muscle or subcutaneous tissue), the skin is more readily accessible and replete with a vast repertoire of immunocompetent cell types (e.g. dendritic cells) whose activation is considered a primary and essential step in effective immunisation. Additionally, the rich innervation of vascular and lymphatic vessels ensures rapid trafficking of immune cells and other macromolecules (e.g. biologics) to the wider circulatory system. These features translate into numerous, well-established benefits, including:
- For vaccines, functional improvements in the durability, strength and type of immune response following vaccination
- Non-inferior immune responses with fractional dosing (i.e. dose sparing)
- For biologics, improved pharmacokinetic profiles (increased bioavailability, faster onset of action, increased Cmax/Tmax, etc)
- Reduced needle phobia from shallow needle penetration.
“The dermis, however, due to its finite thickness, superficial anatomical location and viscoelastic properties, poses additional challenges that have yet to be adequately addressed.”
Drug Delivery Limitations Typically Associated with Intradermal Delivery
Despite these benefits, however, ID remains a chronically underused route of administration (ROA), underserved by a lack of efficient, reliable and versatile delivery device technologies or injection methods. For example, the Mantoux technique (clinician inserting a needle into the skin at an angle) – first described more than 100 years ago – still remains the clinical standard for performing ID injections.
This procedure is technically challenging to perform, as the approach angle and depth of needle penetration are user-dependent and therefore subject to a high degree of variability. In fact, it has been demonstrated that up to 70% of ID injections administered using this technique are delivered to the incorrect depth (i.e. not intradermally).1 Subsequently, a number of drug delivery technologies have been developed over the years to improve the ease of dermal delivery (e.g. jet injections and hollow, hypodermic and microneedle systems), however, these have achieved limited clinical and commercial success. Often, they struggle in some or all of the following areas:
- Reliably administering to the correct depth in the skin
- Regulatory concerns over consistent dose administration
- Lack of confidence for the administering healthcare professional (HCP)
- Typically very low volume and viscosity capabilities
- Difficulties around scale-up and manufacturing.
INTRODUCING THE PHARMA LATCH HOLLOW
The Pharma Latch Hollow (PLH) is an intradermal injection device based on the “Latch” platform of opposing angled hypodermic needles. This novel injection platform facilitates simple, precise and repeatable intradermal injections, allowing the ID ROA to be a truly viable option for the pharmaceutical industry for the first time. Furthermore, the combination of a unique fluid pathway and favourable manipulation of the biomechanical properties of the skin, allows the Latch platform to inject high volume and viscosity formulations (up to 3 mL, 60 cP), increasing the clinical applications of ID delivery beyond what was previously thought possible. This novel injection platform facilitates the development of a pipeline of future autoinjector and self-injection combination products (currently under development) that can address the complex delivery challenges of therapeutics for the management of chronic diseases.
THE ID DELIVERY PROBLEM
For an ID injection to be considered successful, it must achieve full penetration of the needle lumen to a precise depth in skin. Furthermore, the position of the needle must be maintained during the injection in order to ensure complete dose delivery to the intended depth and avoid leakage. Similar principles apply to intramuscular (IM) and subcutaneous (SC) injections but, in those cases, the physical target tissue is sufficiently bulky and deep from the skin surface – easily satisfying these targeting requirements. The dermis, however, due to its finite thickness, superficial anatomical location and viscoelastic properties (that are inherently resistant to puncture), poses additional challenges that have yet to be adequately addressed.
Current convention for performing ID injections uses a combination of techniques and devices that can be grouped into three main categories, as follows:
- Using a 27G needle (i.e. Mantoux technique)
- Employing devices exhibiting fixed-length needles applied perpendicularly to skin
- Using microneedle-based devices applied at an angle to the skin surface.
Figure 1 illustrates the challenges posed by the skin with each approach. In each case, application of the needle/device to the skin is associated with uncontrolled skin compression and deformation, leading to the establishment of skin “tenting”. Effectively, this relates to the extent to which skin deforms before and during needle puncture. This tenting phenomenon further compromises the ability of the clinician to precisely and repeatably reach the target depth, as the underlying layers of the skin are also compressed or “sandwiched” together.
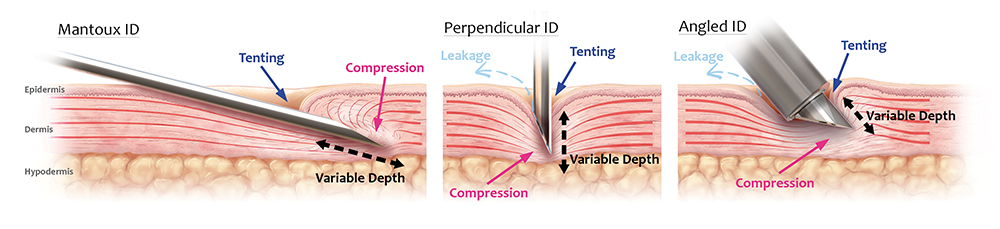
Figure 1: Overview of conventional approaches to ID injections. Left: Mantoux technique using a 27G needle. Middle: Fixed-length hollow needle applied perpendicularly to skin. Right: Microneedle-based device applied at an angle to skin. For all approaches, the viscoelasticity of the skin results in “tenting” and compression, resulting in poor needle insertion efficiency and lack of precise depth control and unconfined skin compression. The ability to administer the full dose to the correct depth and compartment in the skin is further compromised by the requirement for high injection pressures, which can result in leakage and spray-back.
Furthermore, excessive compression reduces the volumetric capacity of the injection site, causing reduced tissue distribution and increased injection pressures. As such, there is an increased likelihood of spray-back or injection site leakage, as well as injection volume limitations. Additionally, tissue compression and increased injection pressure can activate the deep mechanoreceptors of the skin, generating pain and discomfort for the patient. From a usability perspective, mounting injection pressures increase the difficulty of performing the injection, causing significant burden and discomfort to the clinician.
These complications are further exacerbated by factors affecting the mechanical behaviour of skin (including age, race, sex, illness and comorbidities) and by the formulation of the injectate. Ultimately, current injection techniques and devices do not adequately address the biomechanical properties of the skin. As such, they:
- Deliver doses to incorrect depths and compartments in the skin – compression and tenting of skin results in variable targeting
- Must have low viscosity and volume combinations – typically 100–250 μL or less and – are largely only aqueous-based formulations
- Are difficult to administer – significant training is required to perform them and experience is needed to master each approach
- Require significant syringe forces that further complicate usability – high syringe plunger loads are required to pressurise and force the injection into mechanically compressed tissue
- Have high injection pressure requirements that, coupled with skin tenting, can leave the needle lumen open to atmosphere, resulting in a wet injection or spray-back of the injectate onto the patient and/or clinician
- Cause pain and discomfort for the patient – compression of skin activates skin mechanoreceptors, resulting in increased pain and discomfort, further exacerbated by high-pressure fluid flow in mechanically-compressed tissue (hydrodynamic effect).
Accordingly, the potential therapeutic benefits of ID delivery are yet to be realised. In addition to working in traditional vaccine applications, Pharma Latch can be effective in a range of new combinations and next-generation therapies, such as cancer immunotherapies, cell-based therapies, biologics and vaccines for prophylaxis and for chronic conditions.

Figure 2: Overview of the PLH, which is being developed as a single-use, sterile-packed, HCP-administered medical device. The PLH is compatible with both Luer lock and clip syringes, and exhibits arrays of angled 31G hypodermic needles at its distal end for delivery of the injectate into the dermal layer.
SOLVING THE PROBLEM OF ID INJECTIONS
Having identified the biomechanical challenges posed by the skin and the established and emerging drug modalities that could benefit from ID delivery, Pharma Latch developed a hollow-microneedle based technology platform capable of addressing the existing shortcomings of ID delivery. Its first product offering built off the platform – the PLH – is in development as an HCP-administered, sterile-packed, single-use, disposable medical device to be used for ID injection of drugs and vaccines approved for that ROA (Figure 2).
Currently under development as a Class II US FDA medical device (i.e. 510k pathway), the PLH is designed to be:
- Compatible with existing parenteral workflows
- Integrable with prefilled or pre-drawn Luer lock/slip syringe barrels. At its distal end, the PLH exhibits two arrays of three angled 31G hypodermic needles set into the device, all of which are in fluid communication with the attached syringe. Figure 3 depicts the steps involved in administering an ID injection using the PLH.
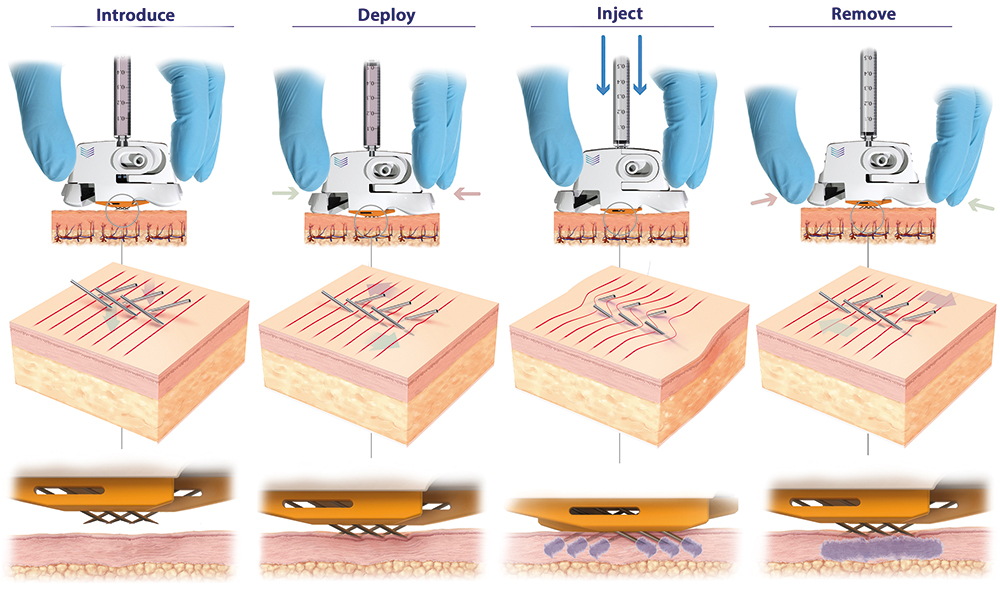
Figure 3: PLH operation (left to right). Device is placed over the injection site. The needles are then deployed using a simple “clicking” action, which establishes a slight stretching of the skin, preventing tenting and compression and enabling full penetration of the hypodermic needles to a predetermined depth (820 μm). Injection can be performed with minimal resistance and with superior diffusion into uncompressed tissue. The PLH can then be removed from the skin, using simple squeezing of the release tabs, and disposed of.
In a first step, the device is placed over the injection site until shallow indentations are formed on the skin, which can be any anatomical site accustomed to receiving parenteral injections. Maintaining this downwards pressure, the needles of the device are then deployed into the skin using a simplified “clicking” or squeezing action. This resulting linear motion of the angled hypodermic needle tips, which are in an initial state of overlap, applies subtle stretch to the tissue, keeping the skin taut and preventing skin from tenting and compressing during needle insertion and propagation.
“PLH provides ease of injection and can work with volumes (>1 mL) and viscosities (up to 60 cPs) that were previously only achievable with IM and SC administration.”
Highly efficient needle insertion mechanics are achieved, requiring low needle insertion forces, resulting in reduced pain and discomfort for the patient compared with existing approaches. At the final step, with the injection administered, by applying an intuitive clicking action to the release tabs, the PLH can be removed from the skin and disposed of accordingly.
This method of needle insertion, unique to the PLH, ensures:
- Precise needle penetration, with the tips of the hypodermic needles, which are at a fixed vertical height from the device, delivered to a predetermined, precise, repeatable depth (circa 800 μm) into the skin with each application
- Dramatically reduced skin deformation and compression, offering a step change in diffusion compared with standard approaches and devices
Low injection pressures and syringe barrel forces, as distribution of the injectate takes place across all six 31G hypodermic needles into uncompressed skin, greatly improving usability.
Internal testing (data on file) for the PLH has shown superior syringeability and injectability compared with a single 27G needle and competitor devices. In terms of syringeability, 4x and 6x reductions in injection back pressure at flow rates of 1 mL/min and 10 mL/min, respectively, using a low viscosity standard (1.2 cPs) were achieved for the PLH compared with a single 27G needle. For the competitor product, the reduction at 1 mL/min and 8 mL/min was 8x and 20x, respectively. Testing had to be stopped for the competitor device at flow rates higher than 8 mL/min due to excessive back pressures.
Further injectability studies in a combination of in vitro and in vivo preclinical large animal tests with several partners (data on file) have confirmed the ability to deliver large injection volumes (up to 3 mL) and achieve superior diffusion and improved usability compared with conventional ID approaches.
In summary, PLH offers an ID delivery device solution that:
- Can deliver the full dose to the correct layer in the skin for every patient
- Is able to work with both low and high volume/viscosity formulation combinations
- Has low injection back pressures, eliminating the potential for leakage and spray-back
- Reduces needle anxiety, pain and discomfort, and improves the patient experience
- Is intuitive, simple to use and easy to administer
- Attaches securely to the skin, providing confidence in administration
- Has a highly scalable manufacturing roadmap, capable of being deployed as a medical countermeasure and futureproofing rapid pandemic responses.
“PLH dramatically simplifies the procedure for the user and removes the variability associated with traditional techniques by having device geometry – and not the user – govern needle insertion depth.”
CHANGING THE ID INJECTION PARADIGM AND OUTLOOK
PLH introduces a new and improved device-based solution for injecting liquid formulations into the skin, achieving this in a number of different ways. Firstly, PLH dramatically simplifies the procedure for the user and removes the variability associated with traditional techniques by having device geometry – and not the user – govern needle insertion depth. Secondly, through a combination of favourable conditions that are established in the skin and inherently low internal fluidic resistance, PLH provides ease of injection and can work with volumes (>1 mL) and viscosities (up to 60 cPs) that were previously only achievable with IM and SC administration. Thirdly, PLH can ensure full dose delivery consistently and repeatedly in all patients, thereby satisfying clinicians and regulators, alike. Finally, compared with conventional approaches, PLH improves the patient experience by reducing needle anxiety, pain and discomfort, thereby improving and driving compliance.
PLH provides a reliable device solution for ID administration that can support and remove the device/delivery risk from preclinical and clinical development programmes. Additionally, PLH is being built on a core platform technology that supports the development of a pipeline of future self-injection and autoinjector products to complement the PLH product. These future Pharma Latch products have the potential to meet the complex delivery needs of therapeutic modalities in development for the at-home treatment of chronic diseases, and where ID delivery is increasingly being recognised as having an important role to play.
To discover more about Pharma Latch, visit the website: www.pharmalatch.com.
REFERENCE
- Micheels P, Goodman L, “Injection Depth in Intradermal Therapy: Update and Correction of Published Data”. J Drugs Dermatol, 2018, Vol 17(1), pp 88–96.
Previous article
ACCELERATING INNOVATION: THE POWER OF PARALLEL DEVELOPMENT FOR AN AUTOINJECTOR LAUNCHNext article
COMPANY SHOWCASE: VETTER PHARMA
