To Issue 158
Citation: Stevens N, “How to Choose the Right Intranasal Vaccine Device”. ONdrugDelivery, Issue 158 (Apr 2024), pp 82–85.
Nia Stevens, discusses the potential benefits of developing vaccines for intranasal administration with a suitable drug delivery device, as well as the challenges associated with this delivery route.
Over the past couple of years, there has been considerable interest in developing intranasal covid-19 vaccines, with several in clinical development globally and a small number approved in countries such as India and Russia. New data have shown the potential for greater efficacy with an intranasally delivered covid-19 vaccine, compared with the standard delivery route of intramuscular injection.
“Providing an at-home administration option for vaccines would help to avoid exposing patients to the risks of sharing a closed space with other potentially infected people.”
This trend is not surprising, considering that covid-19 is a respiratory tract infection, and intranasal vaccination provides targeted local delivery to the upper respiratory airway mucosa. Building immunity directly in the nasal mucosa also offers better potential to prevent disease transmission, as the nasopharynx is the body’s first encounter with infectious aerosols. Early clinical data for intranasal vaccines suggest that they also avoid the side-effects, such as “flu symptoms”, associated with existing covid-19 vaccines.
This growth in interest for nasal covid-19 vaccine delivery follows a wider trend of intranasal flu vaccine development. One vaccine, FluMist/Fluenz Tetra (AstraZeneca), has been available to patients in the US and EU markets for several years now. If a covid-19 intranasal vaccine is successful in reducing transmission rates, where the current intramuscular vaccines cannot, this may become the delivery route of choice in the future of vaccine development, particularly for respiratory tract infections.
There are several other key benefits of intranasal vaccination, including it being a pain-free delivery option that can be administered at home. Providing an at-home administration option for vaccines would help to avoid exposing patients to the risks of sharing a closed space with other potentially infected people, among the other downsides associated with attending health clinics, such as the inconvenience or accessibility barriers of travel. Individuals with needle phobia (or other anxieties about the perceived invasiveness of an injection) may also be more likely to accept an intranasal vaccine.
While the benefits are clear, numerous challenges remain. One important question for vaccine developers is “How do you choose the right device to deliver your intranasal vaccine?” To answer this, it is important to first consider the challenges that intranasal delivery brings.
“Intranasal vaccine delivery introduces a comparatively high dose variability, resulting from the challenge of delivering the dose into the nasal cavity through the narrow nasal valve.”
WHAT CHALLENGES ARE ASSOCIATED WITH INTRANASAL VACCINE DELIVERY?
Despite its advantages, intranasal vaccine delivery introduces several challenges compared with injection. To begin with, there are several compliance and adherence challenges around the use of a nasal vaccine during a pandemic. For example, if users are given an intranasal vaccine to administer at home, how can public health authorities be confident that the vaccine was taken and that an efficacious dose was delivered?
In addition, there is a need to develop ways of measuring the local immune responses in the nasal cavity to evaluate vaccine efficacy. This is because immune responses in the blood plasma, normally used to assess vaccines, are typically low for intranasal vaccines and are not the most relevant or important efficacy measure for an intranasal vaccine. Rather, the level of intranasal immunity is more important with regards to preventing infection by or transmission of a disease. This requirement for such novel methods increases development costs and timescales, and also adds to a project’s technical risks.
Another key challenge, from a medical device perspective, is dose delivery. Intranasal vaccine delivery introduces a comparatively high dose variability, resulting from the challenge of delivering the dose into the nasal cavity through the narrow nasal valve. Factors such as droplet size, plume shape and velocity, length of actuation stroke, angle and depth of insertion into nasal cavity, as well as nasal cavity geometry and mucociliary clearance rates, all impact the delivered dose.
HOW TO TARGET AND MAXIMISE DOSE DELIVERY FOR AN INTRANASAL VACCINE
The nasal lymphoid tissue (NALT) is generally considered to be the primary target for intranasal vaccine delivery.1 This comprises an area of lymphoid follicles at the back of the nasal cavity near to where it meets the oropharyngeal cavity. One key challenge with intranasal delivery is that mucociliary clearance can typically clear the cavity over the course of around 20 minutes, which means any vaccine has a limited time to work; to combat this, mucoadhesive excipients are often used in nasal formulations to improve retention in the nasal cavity. Another approach is to increase the viscosity.
Traditional nasal sprays deliver a dose to the front of the turbinates, which is then transported by mucociliary clearance to pass by the rest of the nasal turbinates and the NALT (Figure 1). Mucociliary clearance is, therefore, both friend and foe in intranasal delivery. To ensure delivery to the internal nasal cavities, including the turbinates and NALT, the dose needs to pass through the narrow nasal valve opening. Any dose deposited outside of the nasal valve will drain back through the nares (nostrils) and be lost.
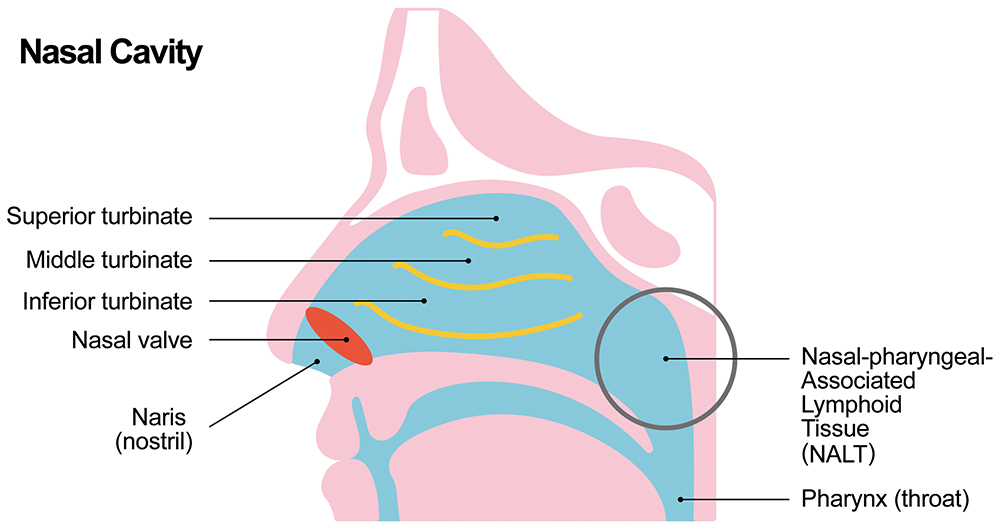
Figure 1: Nasal anatomy.
“It may also be beneficial to evaluate deposition within a nasal cast either via experiments, CFD modelling or both.”
Droplet size has a big impact on where the dose is deposited.2,3 For example, droplets smaller than 10 μm may pass through the nasal passages to the lungs, where they may contribute to local, unwanted side-effects. Meanwhile, droplets around 10 μm in size deposit evenly across the turbinates, although there is an additional concentration of dose at the front of the nasal cavity with some lost through the nasal valve. Increasing droplet size to 50 μm, for example, shows increased concentration at the front of the turbinates, but also a loss through the nares.
In addition to droplet size, the spray plume angle will affect the dose delivered through the nasal valve into the nasal cavity, meaning this also needs to be taken into consideration when selecting or developing a nasal delivery device.2,3 The orientation of the spray nozzle in relation to the nasal valve – the angle of insertion – has been shown to have an impact on the dose delivered to the nasal cavity.2,3 If the nozzle is out of alignment with the nasal valve, a greater proportion of the dose is lost by draining back through the nares.
Lastly, the impact of fluid shear on any vaccine potency should be carefully considered and evaluated to maximise the efficacious dose. High fluid shear rates can damage delicate biological entities, such as vaccines.
IMPACTS OF DEVICE DESIGN ON VACCINE DELIVERY
Various device options exist for intranasal delivery that could feasibly be used to deliver vaccines, with aerosolised liquid spray pumps currently dominating the market. Most relevant to vaccine delivery are unit- and bi-dose (one for each nostril) formats that typically deliver a pre-metered dose. Other options include dropper, pressurised gas canister and dry powder devices.
Nasal Pumps
Most nasal pumps deliver a polydisperse spray with a median droplet size of around 30–80 μm, which is delivered to the nasal valve area and the front of the turbinates.3 The spray is typically formed via either a plain nozzle orifice or swirl atomisation. The nozzle orifice and swirl chamber geometry all play an important role in controlling the spray formation and its characteristics. It is worth noting that both the device and formulation viscosity will affect the droplet size and the resulting deposition patterns. The addition of mucoadhesive or other excipients that increase the viscosity will result in larger droplets and a reduction in the dose delivered to the nasal cavity. A trade-off therefore exists between mucoadhesive properties and deposition.
Soft Mist
Soft mist nasal delivery devices can deliver well-controlled, small, slow-moving droplets using a technology originally developed for lung delivery. Soft mist inhalers refer to technologies generating smaller, slower droplets either via a colliding jet or passing the fluid through an array of micro-orifices. The micro-orifice array delivery system is available as an intranasal format. This generates well-controlled droplet sizes at low fluid velocities, allowing for a more even deposition across the intranasal cavity. Additionally, this lower energy droplet generation method is low shear, which may be beneficial in protecting the vaccine from damage and degradation.
For example, one study comparing a version of this soft mist technology for inhalation delivery, with standard jet and vibrating mesh nebulisers, resulted in less damage to the SARS-CoV-2 mRNA vaccine when delivered using the soft mist device.4 It is possible that such advantages may translate to vaccine delivery using a soft mist device compared with intranasal spray pumps.
Nozzle Stem and Alignment
The form of the nozzle stem is also critically important, since this influences how well the spray orifice is aligned with the nasal valve in the nostril. The stem must be sufficiently long and narrow to fully enter the nostrils, but not so long and narrow that there is a risk of it being placed in the nasal valve itself. To tackle this, it is important to design a suitable ergonomic stem and provide adequate user information on dose critical steps, such as angle of insertion.
HOW CAN INTRANASAL DEVICE PERFORMANCE BE EVALUATED?
There are several methods available to evaluate intranasal device performance for delivering a vaccine. Droplet size can be measured using laser diffraction, a light scattering particle measurement technique. Meanwhile, spray plume shape can be evaluated using laser imaging of the spray, normally carried out in axial and transverse planes for plume angle and spray pattern measurements. Alongside shot weight (measured gravimetrically), these are all mandatory tests for intranasal products for regulators such as the EMA and US FDA.5
It may also be beneficial to evaluate deposition within a nasal cast either via experiments, computational fluid dynamics (CFD) modelling or both. This test is not formally required by regulators, but provides valuable information about the distribution of the vaccine dose within the nasal cavity and distribution across different regions, such as the nasal valve, turbinates and NALT.
The impact of nozzle alignment and insertion depth on dosing can also be evaluated. For example, this may be done in conjunction with user studies to evaluate nozzle insertion for a representative user group. CFD enables researchers to gather detailed information that is not provided by experiment, whilst experimental data can help validate the CFD model.
For a vaccine, potency evaluations will also be required. For an intranasal vaccine, the potency of the vaccine following delivery will need to be evaluated and any impacts of the delivery device on potency understood and controlled. Finally, CFD can help to evaluate fluid shear rates during delivery to select a low-shear device or optimise device design to minimise shear.
HOW CAN INTRANASAL VACCINE DOSE DELIVERY BE OPTIMISED?
The challenges of intranasal vaccine delivery open up a number of opportunities. For example, ergonomic device features could help to reliably control angle and depth of insertion into the nostril. Targeting a reduced droplet size of around 15–30 μm may help to reduce dose loss through the nares. Design innovations could also support patients in actuating a full stroke and delivering a full dose, while ensuring consistency of delivery velocity. As is the case for injection and pulmonary drug delivery devices, onboard digital sensors may also open up valuable opportunities to track patient compliance and confirm if a dose has been delivered correctly.
Developing an intranasal covid-19 vaccine could bring considerable benefits, if it can be shown to reduce disease transmission in a way that intramuscular injections cannot. It goes without saying that this breakthrough would bring high potential payback for investments in these endeavours.
To boost the chances of a positive outcome in intranasal vaccine clinical trials, it is important that they are supported by suitable delivery devices. Applying the right device test programme and, if needed, design modifications, can maximise the chances of success.
REFERENCES
- Ramvikas M et al, “Chapter Fifteen – Nasal Vaccine Delivery” in “Micro and Nanotechnology in Vaccine Development” (Skwarczynski M, Toth I, eds). Elsevir, Micro and Nano Technologies, 2017.
- Tong X et al, “Effects of nasal drug delivery device and its orientation on sprayed particle deposition in a realistic human nasal cavity”. Comput Biol Med, 2016, Vol 77, pp 40–48.
- Kimbell JS et al, “Nasal steroid spray simulations using measured spray characteristics in healthy and rhinitic nasal passages”. J Aerosol Sci, 2023, Vol 174, Article 106246.
- Van Rijn CJM et al, “Low energy nebulization preserves integrity of SARS-CoV-2 mRNA vaccines for respiratory delivery”. Sci Rep, 2023, Vol 13(1), Article 8851.
- “Guidance for Industry: Nasal Spray and Inhalation Solution, Suspension and Spray Drug Products-Chemistry, Manufacturing, and Controls Documentation”. US FDA, Jul 2002.

