To Issue 128
Citation: “Interview: Arnaud Guillet, Biocorp”. ONdrugDelivery, Issue 128 (Dec 2021), pp 26–32.
In this exclusive interview with ONdrugDelivery, Arnaud Guillet discusses Mallya, Biocorp’s connected clip-on for pen injectors, including Mallya’s successful launch in multiple countries this year, the factors that have played into the device’s success, and the future for Mallya, Biocorp and connectivity in the drug delivery industry.
“Rather than design a whole new device with all this built in, we wanted to capture that data with a wide variety of different pens, which means dealing with a variety of designs and geometries – and we’ve achieved it with a single technology that leverages electromagnetic sensing technology.”
Q Regular readers will likely already be familiar with Mallya, but as a refresher, please could you provide a brief introduction to Mallya and how it fits into Biocorp’s overall offering in the connectivity space?
A At the most basic level, Mallya is a clip-on device that turns regular pen injectors into a connected solution (Figure 1). Mallya captures the dosage, and time and date stamps each time the injector is used, with the highest level of accuracy. It can differentiate priming from an actual injection, and confirm that the dose selected was actually triggered by the patient. However, rather than design a whole new device with all this built in, we wanted to capture that data with a wide variety of different pens, which means dealing with a variety of designs and geometries – and we’ve achieved it with a single technology that leverages electromagnetic sensing technology. Mallya is able to capture the critical information we want on various lines of pen injectors and send that via Bluetooth to a companion app or other related software.
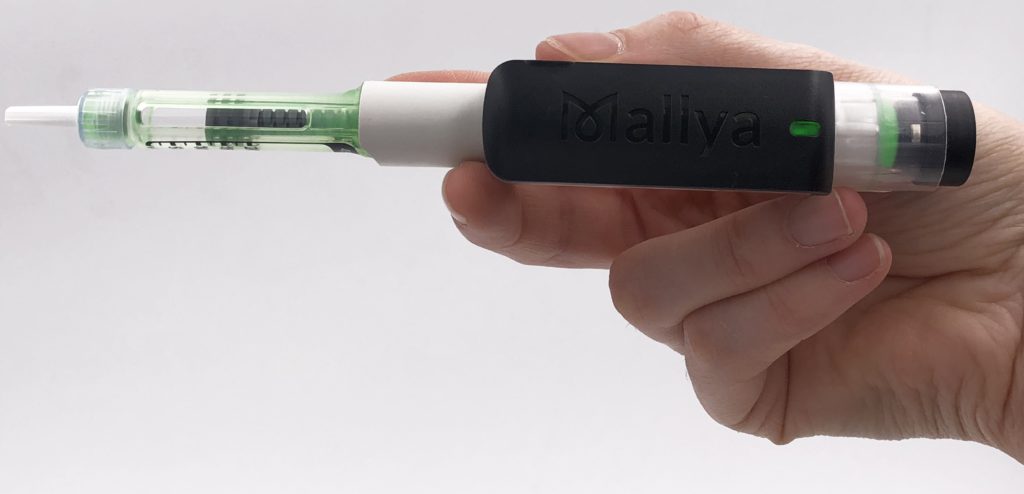
Figure 1: Biocorp’s Mallya, a connected Bluetooth add-on device for pen injectors, which was commercially launched for diabetes in 2021.
The first one is Injay, which is a solution to bring connectivity to regular prefilled syringes (PFSs). Injay is, in essence, a combination of two components: a customised piston rod with a near field communication (NFC) tag for storing product info and a customised finger flange featuring an activator for detecting a complete injection. So, in practice, Injay records the key product information, such as drug concentration, batch number and expiry date, and confirms that the injection has been completed. The data can then be time-stamped and transferred to a smartphone via NFC. We have also developed a variant of this technology that is compatible with PFS equipped with needle safety systems, keeping the same simplicity and value proposal. Biocorp’s goal here is to position itself as the leading player for solutions that bring connectivity to regular drug delivery devices. The central principle of the add-on approach is that it produces easy-to-use, reliable and cost-effective solutions to connect drug delivery devices. Mallya is Biocorp’s top add-on product, but we have two other products that really illustrate this.
Looking at a completely different delivery route, another example is Biocorp’s Inspair device, which is a smart cap for pressurised metered dose inhalers (pMDIs). With Inspair, we not only collect data on the actuation of the device with time and date, but we also want to collect information on the quality of the inhalation technique – using a pMDI correctly frequently proves to be difficult for patients, so if we can give them real, tangible feedback on their technique that’s hugely valuable. Specifically, it’s co-ordinating actuating the device with their inhalation that patients tend to struggle with, which has a major impact on the efficacy of the device if not done exactly right. We collect this information and send it to a mobile app so that patients can review it and, if they want to, share it with their HCP.
These two products are not commercialised yet, but are in a mature stage of development and we care getting good traction from the market.
“We have to make it smart, make it easy and make it commercially viable, all while ensuring that it’s acceptable for users as well.”
At Biocorp we develop one specific device for one specific area. While there are some transferable ideas, you have to approach each device and mechanism on its own terms; with pen injectors, for example, we know that we need to collect accurate dosage information with an exact time and date because this information is critical for insulin management – you have to follow your units carefully, so this is a key functionality Mallya focuses on. Whereas, with Injay and PFSs, recording the exact dose delivered doesn’t really matter – PFSs contain a single fixed dose, so the question is whether you fully inject it or not.
These specific characteristics also impact the choice of connectivity technology we use for each device. For instance, Mallya uses Bluetooth Low Energy (BLE), which is relatively expensive when compared with its peers. However, because Mallya is designed to be transferred between disposable pen injectors for two years or more, BLE is actually the cost-effective option. Injay, on the other hand, uses NFC because it’s cheap and incredibly simple to integrate into a PFS. So, as you can see, it’s not always the same recipe; we have to make it smart, make it easy and make it commercially viable, all while ensuring that it’s acceptable for users as well. For instance, making Injay something you swap between devices every time is a no-go. It’s one thing with a pen injector which you’ll use multiple times, but with a one-and-done PFS this is not going to work.
Q Looking back at 2021, it’s been a busy year for Mallya, and an incredibly successful one, with major deals both in diabetes and other areas. Can you give us a rundown of the year’s Mallya highlights?
“First of all, 2021 has been the launch year for Mallya in the diabetes field, which is massive – we’re hitting the market in both Europe and the rest of the world.”
A First of all, 2021 has been the launch year for Mallya in the diabetes field, which is massive – we’re hitting the market in both Europe and the rest of the world. I’d say one of my highlights there was the initial launch of Mallya back in May with Roche Diabetes Care France, our local distributor. Mallya became available in French pharmacies and connected to the Gluci-chek app, delivering a really great service to patients, who could their insulin injection data from their Mallya, their glucose data from their Roche Accu-Chek device and see it all together in their Gluci-chek app. It was a very exciting launch with a strong value proposal for patients, and we had a great partner in Roche Diabetes Care.
Another highlight was the Taiwan launch with Sanofi. In this case, we were leveraging the Health2Sync platform, which is a famous diabetes management platform that’s already widely used in Asia – specifically in Taiwan, with hundreds of thousands of users. The app is well implemented in clinics and hospitals, so we launched Mallya connected to their platform, and we already have a lot of patients on board, communicating with their doctors on a daily basis to analyse the data and receive recommendations.
“First and foremost, I think that the user-friendliness is an obvious factor. But I think that, if we look back and compare Mallya with its peer competitors, the key to Mallya’s success on the device side is its focus on one key function – the dosage timestamp.”
These launches have also been supported by collaboration with software partners, which is one of the things we really wanted to have in place. We don’t plan to reinvent the wheel, there are already a number of great software platforms in the diabetes space that are used by patients on a daily basis, so we really wanted to integrate Mallya with these existing platforms. We initiated this process with AmalgamRx last year and have reinforced our netowrk this year through integrations with Health2Sync, which I mentioned before, and Social Diabetes, which is very good in Europe and Latin America and features the SmartBolusCalculator, which is a very helpful tool for patients. With these two integrations, we have the potential to reach nearly one million users. Of course, that’s not going to materialise overnight, but we have a step-by-step approach in place and the potential is there, which is really exciting.
And then there are the great partnerships we’ve made with major insulin manufacturers this year. We’ve strengthened our relationship with Sanofi and made progress on specific development of Mallya for its pens, which is going really well, to the point that we’re expecting a lot of launches in the upcoming months. We’ve also signed a deal with Novo Nordisk to adapt Mallya for its FlexTouch pen, which is a very specific pen requiring a totally new approach, and we’re partnering up with Novo Nordisk to develop it further and push it to market.
The last very important highlight of 2021 was our first major partnership outside of diabetes. We’ve partnered with Merck to develop Mallya for its growth hormone applications, which is very exciting because Merck is a pioneer in the connected drug delivery device area. It has been very ambitious with its connectivity strategy – EasyPod Connect launched a few years ago, which is really early in the connectivity space. Merck has real knowledge, expertise and understanding when it comes to patient needs and connectivity, so it’s a privilege to work with the company on a solution for its growth hormone applications. We’re very excited about this partnership, and I think it’s going to be a massive help for patients and HCPs. We also have intense internal discussions and active programmes going on in other therapeutic areas outside pen injectors that I’m really excited about, but I’m not able to disclose any details on those just yet.
Q What do you think are the features of Mallya that explain this success?
A First and foremost, I think that the user-friendliness is an obvious factor. But I think that, if we look back and compare Mallya with its peer competitors, the key to Mallya’s success on the device side is its focus on one key function – the dosage timestamp. It’s tempting to try and cram in as much functionality as you can think of, for example maybe I want the temperature, and I also want the angle of injection, and then there’s always another thing and another and another. Instead, we sat ourselves down and asked, “What exactly do we want to achieve here?” The answer to which is that we want to help patients keep track of their injection data, and what really matters there is what dose is injected at what time, so we focused on just capturing that, and did it very simply.
The result of this approach is that, for pharma companies, implementation of Mallya doesn’t require any modification of their regulatory dossier or their pen and, for patients, there’s no modification to the user process. Once you attach Mallya and pair it all with your phone, there’s no more you need to do, it’s totally passive, totally effortless for the patient. With that key functionality and simplicity, we can really work on the user-friendliness. That’s really the key, I think, Mallya does one thing very simply and very well.
Of course, in Mallya’s case doing “one thing very well” means measuring the injected dose very accurately, which is where we leverage the magnetic technology, because it provides, by far, the highest level of accuracy. Using this technology, we’ve reached very close to 100% accuracy, which has been demonstrated through verification testing. It’s extremely reliable and reputable technology that has made us successful with benchmarking, which I think has been a significant factor for the companies who’ve partnered with us. Really, it’s a key element for all stakeholders, pharma partners, patients and, of course, HCPs – if they’re going to base healthcare recommendations off the data Mallya is providing, it absolutely has to be accurate. Add in the user-friendliness and simplicity we discussed before, and I think you have a good picture of why the market has really embraced Mallya.
Then there’s also the compatibility – Mallya is easily replicable from one pen to another. Of course, you have to adapt to the different geometries of the pens, the specific movements of the knob, that sort of thing, but we always have a solution for that. What never changes is that guarantee of accuracy, the principle of detection using magnetic sensing technology. Through all the programmes we’ve worked on, we’ve pretty much seen all the different form factors, which means we’ve developed a really solid understanding of all the various pen injector mechanics and we can transfer the Mallya technology easily from one to another and provide the same guarantee of accuracy.
“With Mallya, we brought our own software solution, which was a just a basic mobile app, but we built the system so that it could integrate very quickly with any partner’s system directly and flawlessly.”
Q How about on the software side of things? Can you give us some insight into what the demands are on that aspect of connectivity, and which stakeholders are driving it?
A Here my thinking is mostly defined by the diabetes sector, which is where our focus has been for the launch of Mallya. I would say that interoperability and flexibility are the things you really have to deliver. So, with Mallya, we brought our own software solution, which was a just a basic mobile app, but we built the system so that it could integrate very quickly with any partner’s system directly and flawlessly. This approach made sure that we didn’t cut ourselves off from opportunities just because we wanted to push our own software. As I mentioned before, there are plenty of really good software platforms already in use in the diabetes space. Instead, when we talked to our partners, we could say that we have some assets that they could leverage if they wanted to, but if they had their own system we could integrate with it – we can propose our system, but we never push it. We don’t present Mallya as a device-and-software package that’s all or nothing; we’re always open to integration with other software. With diabetes in particular, the software market is both well established and crowded, so we’re focusing development of our own assets on other therapeutic areas where partners are less likely to have their own system already set and decided upon.
As for which stakeholders have the most influence, it really depends on the system. Sometimes you’ll have a pharma company that has invested a lot in its own internal platforms and wants to leverage these assets, and so they ask us to integrate with their assets specifically. This is usually as part of their own business strategy priorities – control of the data and the value chain – which means Mallya needs to be integrated into their system. Most of the time, however, in the diabetes space in particular but also more generally, it’s really driven by the patients and the users, which means working with the solution best positioned in the specific market you’re looking at. Health management software is most widely used by patients and HCPs, so the solution they prefer is the best one to pick.
A perfect example is Taiwan. Taiwan’s best solution is, by far, Health2Sync. It’s well connected with both clinics and patients, so we leveraged that and it’s proven to be successful for the three parties involved: Sanofi, Biocorp and Health2Sync itself.

Figure 2: With its successful commercial launch, Mallya is now in the hands of patients as part of their day-to-day lives.
Q Ultimately, drug delivery device development is about patients, and adding connectivity is no exception to this. Can you share any feedback from patients on Mallya, or from studies that have illustrated how patients respond to and engage with Mallya?
A We’re actually at a really good point to assess this because, now that Mallya’s been launched, it’s in the hands of patients out there in the real world (Figure 2). Before launch, all we had were results from our human factors studies that we collected as part of our typical development process, but those had already given us some interesting information regarding the absence of risk in using the device together with the pen and how well patients were able to understand how to install it, pair it, use it and understand the feedback it generated. That was very positive information for us, it was something we really focused on with Mallya. However, when we reached the market, we weren’t dealing with clinical or supervised environments, instead we’re dealing with thousands of patients in a whole host of settings.

Figure 3: The diabetic community has embraced Mallya, with influencers such as Ines Nerina (pictured) discussing Mallya and posting reviews online.
“We’ve been very positively surprised by what we’ve been getting back, because Mallya is fairly new in terms of what it does and how it’s used, but what we’re getting back is that this was something that patients have been looking for and they specifically appreciate the fact that Mallya is interoperable with other devices.”
So, with Mallya now out there in the world, we’ve received a lot of very valuable feedback, including testimony from patients and beta testers, also influencers have posted reviews of Mallya on the internet, including on social networks and YouTube (Figure 3). We’ve been very positively surprised by what we’ve been getting back, because Mallya is fairly new in terms of what it does and how it’s used, but what we’re getting back is that this was something that patients have been looking for and they specifically appreciate the fact that Mallya is interoperable with other devices. For example, a key point has been the ability for patients to combine their glucose data with insulin readings, enabling them to have meaningful conversations with their HCP. It’s always a concern when you market an add-on device whether it’s going to be an issue for patients to put on, take off and put back on, but the feedback we’re seeing from patients really confirms the fact that we’re creating value for them (Box 1).
BOX 1: FEEDBACK FROM PATIENTS
Quotes from Mallya users, as posted on Biocorp’s website:
“I almost regret not using an insulin pen anymore.”
– Virginie, 29 (Type 1)
“I am far less stressed since I’ve been using Mallya.”
– Serge, 62 (Type 2)
“I really like the device.” – Laura, 24 (Type 1)
“This new medical device does most of the work for me.”
– Mélanie, 35 (Gestational)
And testimony from lifestyle blogger Ines Nerina:
“I have been living with Type 1 diabetes for five years and I have tested many medical devices designed and developed for the management of diabetes. Today, I can say that Mallya is by far a revelation – everything is easier with Mallya! Indeed, one of the most difficult tasks in the routine of a patient living with diabetes is to remember what time the injection has been made, how many units were selected for injection and sometimes whether you have already done your insulin injection or not. This smart device is totally changing the lives of patients with diabetes and my only regret is that I didn’t discover Mallya sooner.”
There’s one specific example I really love – we did a clinical study in the UK with Dr Edward Holloway from the Croydon Health Institute, and he made a presentation about the solution. He said that Mallya enabled him to spot specific gaps in the treatment of a patient that he was following, whose diabetes wasn’t well controlled and they couldn’t figure out why. So they equipped Mallya, and Dr Holloway could see the patterns and he found some issue with this patient’s behaviour related to specific issues, which he was able to discuss with them and improve the management of their diabetes.
That study was first conducted in the context of covid-19, so you can imagine how hard it was, but getting that kind of feedback was a really great motivator for us. In terms of direct feedback to us, with the exception of business-to-business distribution, the customer support is all handled by our distributor, but it’s been very quiet in terms of complaints. I would say that wherever we have had issues, we’ve had a good solution and good troubleshooting to identify them, so we’ve be able to then solve these issues very quickly. Overall, we are very positive, although we need to be careful, as it’s only the first 1,000 patients, and the more patients we have, the more challenges we face, but still it’s very encouraging I would say.
“The challenge for the industry is really to educate payers and
market access people so that they can understand the real value of these digital solutions and how we should evaluate them, the digital ecosystem as a whole and its specific components.”
Q We’ve discussed 2021, so now let’s turn to the future. What’s next for Mallya in 2022 and beyond?
A That’s a great question. Let’s focus on Mallya first, when it was first launched it convinced us that we’ve got the right approach, it got a really positive response, but now it’s a matter of increasing general awareness of smart pens, including with respect to getting reimbursements. I think this is really the roadmap for mass adoption because the value of smart pens wasn’t obvious a few years ago, but now there are more and more publications around this value and there’s more and more need to hear directly from patients and HCPs about it. We need to actively support these efforts, but I think this awareness is out there, and growing. Overall, it’s a really positive place to be.
The second part to building a strong roadmap for mass adoption is reimbursements. As such, we are already working with our partners – our distributors, pharma companies and software partners – to build the relevant ecosystem to optimise the user experience, and therefore the value of the product, demonstrating clinical benefits in collaboration with HCPs. Reimbursement options for digital health solutions are maturing in various markets, such as with new current procedural terminology (CPT) codes in the US, digital health applications (DiGA) framework in Germany and the ETAPES programme in France, opening new possibilities for Mallya.
Q Thinking about potential markets, currently the nature of connected technology tends to restrict connected devices to wealthier and more developed countries. So, taking Mallya as the example, do you think that, as time goes on, production factors might mean that the price point can drop to the point that other markets, such as China and India, open up?
A That’s a very good question. In China, we’re also dealing with specific cultural aspects. Sticking with diabetes as the example, there’s a lot of education and awareness you need to put in place with patients first, such as on the importance of adjusting your dose based on your glucose. Then we need to adapt to the specifics of the local digital ecosystem. For instance, in China, everything goes through WeChat, so connectivity with that system will be a key factor, plus you need to integrate with local hospitals and clinics. That’s a challenge. On top of that, there’s a lot of pressure on the price of insulin in China right now. That being said, the needs generated by diabetes don’t change with geography, plus China is a big market with a very digital-friendly population, so it’s not all negative, we just need to go steadily, step by step.
Thinking about price points, as I mentioned, the price of insulin in China is an issue so, we need to leverage everything that we can to decrease this price point and optimise it, as well as doing the same for the device itself. This is also required in India, and potentially even more so. It’s something we’re currently studying.
Q Lastly, let’s broaden the scope a little. Thinking about Biocorp as a whole, and connected drug delivery in general, what do you see coming up on the horizon?
A For Biocorp, I think the first thing we need to do now is prepare for mass production of Mallya. We have the right products and are well positioned with strong partners, so we’ve got a really strong footing for true mass production of Mallya. I would say that’s the big industrial challenge ahead of us.
Talking more broadly, what we want is to transition Biocorp a bit more from being seen as just a device provider to a full solution provider, meaning device plus digital ecosystem. That doesn’t mean we’re pivoting to saying you have to use our system, we’re still committed to integration, however, our pharma partners expect us to bring a solution to the table. It’s about being able to deliver on our partners’ requirements, sometimes they have their own digital solution, sometimes they don’t and sometimes they’re somewhere in the middle and have some local things that they want to leverage at a global level, and they want us to bring an understanding of end-to-end systems and how to take a project from A to Z in the digital space. It is very important to us to be able to cater to all of these. We already have a good position with device players where we work with them from design to manufacturing. I think we should have the same approach and mindset for connected solutions, going from the device design to when the data touches the digital ecosystem, mobile app or web client. That’s what we’re aiming for, and we have the expertise and the ability to achieve it. There’s also potential in some markets for us to go agnostic and go business-to-business-to- consumer or directly business-to-consumer.
As for connectivity in the industry overall? I think it’s very interesting what’s happening right now. I think that covid-19 has really changed the game because there are a lot of patients who, under normal circumstances, were never going to use any kind of telemedicine service, connected device or mobile app to monitor their disease. However, as covid-19 forced the issue, they’ve tried out this sort of device and, in most cases, judging by the data that we collect, they actually enjoy it and find it a reliable benefit.
For chronic disease management, there are many valuable connected solutions out there, it’s just a question now of scaling up, by demonstrating clinical benefits and obtaining reimbursements.
I think that the challenge for the industry is really to educate payers and market access people so that they can understand the real value of these digital solutions and how we should evaluate them, the digital ecosystem as a whole and its specific components. There are a number of questions that need answers in this area; for example, what’s an appropriate reimbursement for the digital ecosystem? What’s the reimbursement for the Mallya components? There are programmes in the pilot phase in some countries that are now going full speed. For example, in France there is the ETAPES programme that I mentioned earlier, which is set to be transferred to a permanent initiative. So it’s definitely going there. Earlier, I mentioned the US CPT codes, which are very exciting for the industry. I think we’re at a moment where things are really starting to take shape and that will open new doors.
Diabetes has definitely been a driver, and will continue to be, because of all the context in this field. But the whole injector field, I believe, will follow behind because of various factors. If you think about it, we have very expensive drugs combined with adherence issues, so payers want to look into what is taken, or not in some cases, and how effective these drugs are in real-life settings. I’m sure this will also come up in respiratory and nasal delivery. There are a lot of areas where I think the theoretical benefits are there, sometimes having been demonstrated in trials, but now we need to do the hard work of building a body of real-world empirical data.

