To Issue 175
Citation: “Interview with Glenn Svedberg: From Pilot to Scale Manufacturing”. ONdrugDelivery, Issue 175 (Jul 2025), pp 52–56.
Q To begin with, could you give us a quick overview of Nolato and your position in the company?
A Nolato is an international public company headquartered in Sweden; it was founded back in 1938 and we’ve been listed on NASDAQ for 40 years. The company’s revenue is around €1 billion (£850 million) a year and we have around 30 sites in 10 countries globally, roughly 20 of which have a medical certification.
Last year 56% of our revenue related to the life science and medical industries. The rest is what we call “Engineered Solutions”, which cover a number of different identified product segments and market areas. In the medical sector, we split the business into four areas – pharmaceutical packaging, drug delivery systems, medical devices and diagnostics.
Not every one of our sites covers everything and we’re not locked to one particular therapy area. We work with our customers’ products to help them and carry them through from concept to high-volume production. We don’t have our own product platforms – I know that some of our peers have developed their own pumps and autoinjectors, but that’s not something we’ve done. We want to stay flexible, both in terms of technology and scale, and not compete with our customers.
As for me, I’ve been with the company for 18 years. I’m part of Nolato’s executive group management and responsible both for co-ordinating our business development operations within medical solutions and for our technical design centres (TDCs) globally. As we discussed at greater length in our first interview last year,1 my role also includes a sustainability component, which may seem odd at first, but that whole triangle – sustainability, business development and technical – is something you want someone at the centre of because you can’t do much with sustainability unless you have technical and business people involved in making it happen.
Q How does Nolato support its partners from the early design stages through to high-volume manufacturing?
A We offer an integrated approach that spans all the way from the earliest concept and the design stages through to industrialisation and validated-scale manufacture. This full-spectrum capability ensures a smooth and efficient transition from pilot to high-volume production. We bridge the gap between innovative device design and scalable, compliant manufacturing – helping customers to increase safety, accelerate timelines and bring better solutions to market faster.
“OUR CORE STRENGTH IS DFM SERVICES, WHERE WE CAN BRING IN OUR EXTENSIVE EXPERIENCE AND APPLY MODERN TOOLS, SUCH AS VIRTUAL PROTOTYPING, WHICH LETS US ACHIEVE A LOT UPFRONT BEFORE WE START CUTTING STEEL.”
Our core strength is design for manufacturing (DfM) services, where we can bring in our extensive experience and apply modern tools, such as virtual prototyping, which lets us achieve a lot upfront before we start cutting steel. When we get an initial design, we can use virtual prototyping software to get a really good look under the hood and help our customers identify pain points in the design before they occur in physical prototyping. For example, we can investigate how locks or hatches are working and use these virtual tools to demonstrate that for the customer before we start to apply a tool strategy, which is very helpful because it enables us to have more robust and productive discussions (Box 1).
BOX 1: NOLATO’S VIRTUAL FACTORY
By using digital tools and combining modelling software approaches, medical device design can be made more robust and reliable before ever making a physical prototype (Figure 1).2 Nolato combines mould flow simulations with measuring software, such as computed tomography (CT) scanning, to model devices and identify design hotspots early, and to iterate on the device throughout development. Taking this approach can help to avoid:
• Project delays
• Tool adjustments
• Moulding imperfections
• Material changes
• Manufacturing defects affecting device functionality
• Delayed process qualifications
• Unpredictable outcomes
• Late and costly design changes
• Inaccurate measurements
• Expensive moulds.
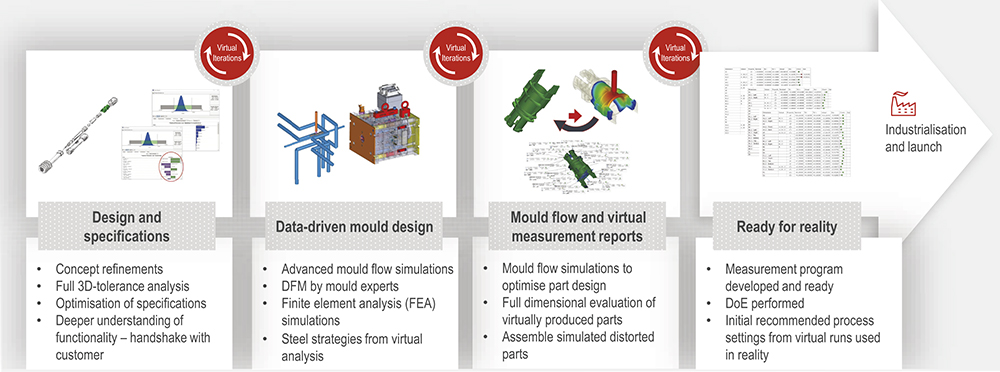
Figure 1: Nolato’s virtual factory process.
At the same time, we make sure to include eco-design principles – by using simpler materials and lower weights, we can make a component that has a lower carbon footprint and is more cost competitive. We also sometimes employ rapid prototyping if needed, but we often only do that as a final iteration step when we’ve had the earlier discussions. And, for a drug delivery device, it’s also critical to involve the quality assurance section.
We ensure that there is a full audit trail from day one that we can supply to our customers for their design history file. Of course, each customer will have their own process so, while we have a general process for product development projects, we are able to adapt it to the needs of the customers. If we have a site that has experience in a certain therapy area or a certain type of device, we do our best to involve them as early as possible to facilitate clear and productive discussions.
Similarly, we try to include any site that’s going to be involved in the development or industrialisation process as early as possible, for example if a US-based company wants our help establishing manufacturing in Europe. We are a decentralised company, so don’t have a lot of centralised engineers. Our TDCs are able to support a given project, but the majority of the work is done by the individual companies that make up Nolato Group. That’s why we see it as pivotal that they have a stake in the project, so they are involved from the beginning.
As an example, just this week I was involved in a project we started some years ago with a Boston (MA, US)-based company. The project was for a device in the insulin field that we worked on some components for at a quite early stage – they wanted to have manufacturing in Asia, so we made some parts in Asia for them. They then came up with a much more complicated technology and they had a Swiss tool maker for the advanced manufacturing process it required, and we happened to have experience with that tool maker for a similar product; it had a completely different application but still used the same basic technology.
So we brought them together and now we will start to scale that up and validate it because it will be quite a long project, maybe a year and a half. Then, when everything is set up and approved, we can transfer the tool to the Asian site.
Typically, we prefer to put processes in place at the target site from day one, but in this case we wanted to work with the tool maker in a closer and more controlled way. That’s how we use our small pieces of the puzzle and combine them in a clever way.
“OUR CROSS-FUNCTIONAL TEAMS SPANNING R&D, ENGINEERING, QUALITY ASSURANCE AND PRODUCTION WORK TOGETHER TO TRANSFER DESIGNS INTO ROBUST MANUFACTURING PROCESSES.”
Q How does Nolato ensure that taking the step from design verification to industrialisation is robust and risk-free?
A An important aspect to discuss is user requirements. Customers can have a very subjective view of their requirements, so for us it’s about getting a feel for expectations, seeing where we can and can’t push, and then converting that into concrete product requirements. That’s the TDC core competence; we can help with establishing requirements at an early stage (Figure 2). Once that’s settled, we get into the core competence of Nolato, which is the single concept selection. If there are several ideas, we can do simulations and highlight the potential risks and different challenges with various solutions, giving customers what they need to make an informed decision about how to proceed.
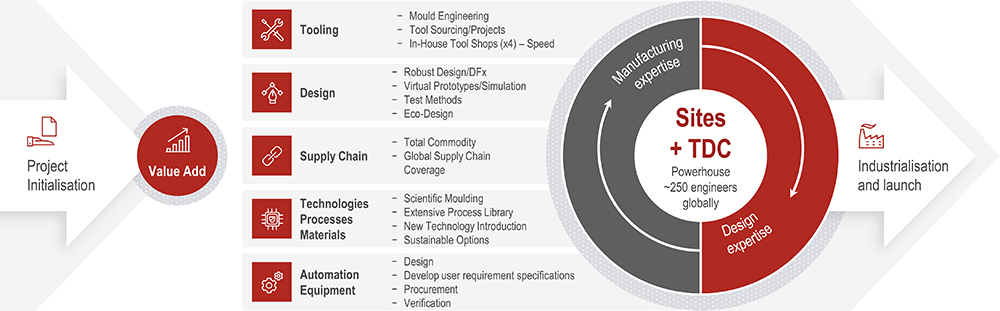
Figure 2: Capabilities of Nolato’s TDCs.
Q What capabilities does Nolato offer to accommodate different production volumes, from clinical to commercial?
A Our infrastructure is designed for flexibility. We support pilot-scale production with semi-automated or manual processes, ideal for clinical and early commercial phases. As demand grows, we scale seamlessly to fully automated, high-volume manufacturing – often in cleanroom environments depending on the requirements. We also design and implement customised manufacturing cells and automation systems tailored to each customer’s device and lifecycle stage. This scalability is a key reason why leading pharma companies choose Nolato as a long-term partner.
Sometimes this transition involves different parts of the customer’s organisation, which can present a challenge. For example, imagine a project-aligned team focused on the early development stages handing over to a production team– because we work with both small and large scale, Nolato can help by carrying information and knowledge between those teams, providing continuity for the project. Other times we’ll be helping communication and transition between different locations.
Another aspect that’s easy to overlook is making sure that scale-up and DfM are considered at every stage, which is core to Nolato’s approach. It can be easy to get lost focusing solely on performance and meeting critical dimensions and tolerances, which can lead to a good and robust device but one that’s difficult and expensive to scale up. You need to think about scaling up from the beginning and continue to keep in mind as you move from single- to multi-cavity tools, all the way up to full commercial scale. Ideally, we always try to do this all within the same factory, using the target site from the very first day through the long term, even if that means a lower quantity to start with.
Q Could you go into some more detail about how Nolato helps to ensure that manufacturing processes are regulatory ready?
A We have a global quality network that is headed by one of the senior quality directors, but, due to our decentralised structure, there is no one single quality system for Nolato – the individual companies have their own quality management systems (QMS) and select what certifications they need given their business profile and customer base, tailoring their approach to best suit their needs. As such, we try to keep overall corporate guidelines as limited as possible.
Having said that, we do have a standard framework in the medical sector that we call the “Medical Excellence Model”, wherein regulatory compliance is baked into our process from the start. Whether it’s for ISO 13485, US FDA or EU MDR compliance, or even customer-specific requirements, our teams are well-versed in designing and validating processes that meet global standards. We develop detailed validation plans and ensure traceability and data integrity throughout. Our customers can rely on us not just for technical expertise, but for regulatory peace of mind as they approach market launch.
We can also use the expertise of certain sites that have experience handling specific interactions with the FDA, for example. There’s a whole language involved with dealing with regulators, so we try to maximise value for our customers by identifying which companies within Nolato have the most experience with the relevant regulatory bodies. For example, we had an FDA audit at one of our Swedish sites handling advanced device modules just two weeks ago. It all went very well and that company used their local QMS, supported by a few of our global guidelines, and there were no issues. Ultimately, it comes down to providing the customer with the best possible support.
“CENTRAL TO THIS IS THAT WE VIEW OURSELVES AS AN EXTENSION OF OUR CUSTOMERS’ TEAMS AND INVEST IN LONG TERM PARTNERSHIPS, NOT JUST SHORT-TERM CONTRACTS.”
Q What makes Nolato a valuable strategic partner rather than just a supplier?
A Central to this is that we view ourselves as an extension of our customers’ teams and invest in long-term partnerships, not just short-term contracts.
Our involvement goes beyond the factory floor – we collaborate on technical strategy, supply chain planning and risk mitigation. Our global footprint really helps us here, as it enables us to cover for gaps in our customers’ capabilities. For example, a customer might have a strong presence in the US and Asia, but not so much in Europe, but we have a footprint in all three of those major regions so can provide the European coverage (Figure 3).

Figure 3: Nolato can provide localised manufacturing for a target market with a global approach that ensures quality and consistency.
Another factor is that we are quite flexible in terms of approving new investments. For example, we had an opportunity in Malaysia, where we have a site manufacturing for Engineered Solutions customers. A lot of our customers these days want to have options in South East Asia for accessing China. We started with a small cleanroom in our existing facility in Southeast Asia with the aim of going to market from there. Then, while that was in progress, we also won some other business in the region and are now considering expanding with a larger site. All within one quarter, we identified a new building and put together the business case, which is likely to go ahead, giving customers real confidence.
“OUR COMPANY CULTURE OPERATES ON THREE CORNERSTONES BE PROFESSIONAL, BE ORGANISED AND TAKE RESPONSIBILITY.”
Then there’s our decentralised structure, which supports that agility and enables us to take decisions much closer to customers and makes our approach unique. This is possible because our company culture operates on three cornerstones – be professional, be organised and take responsibility. This is something that we truly believe in and is part of the Nolato spirit.
Cross-fertilisation between the different product areas within the larger Group is also key to our success. Because ideas flow freely within the company, we can get a clear view of trends across the sectors we operate in, such as miniaturisation, connected devices and increased inclusion of electronics. We can then pull that knowledge from fast-moving sectors, such as consumer goods and mobile phones, and implement what we’ve learned in the medical arena.
As a strategic partner, we can pool all of these advantages and really deliver for our customers. In practice, we’ve found that this strategic alignment is critical for successful device launches for our pharma and biotech partners, and we will continue that journey with them.
REFERENCES
- “Interview with Glenn Svedberg, Nolato Group”. ONdrugDelivery, Issue 165 (Sep/Oct 2024), pp 26–29.
- Ingvarsson P, “Nolato’s Virtual Factory”. ONdrugDelivery, Issue 166 (Oct 2024), pp 96–99.

