To Issue 162
Citation: “Interview with Eric Leven, Rip Road”. ONdrugDelivery, Issue 162 (Jun 2024), pp 6–9.
Q To begin with, can you give us an overview of Rip Road and what role the company plays in the pharma industry?
A Fundamentally, Rip Road is a software developer, although many within the industry would call us a digital health company. Our focus is providing companion apps for biologics and drug delivery devices. We design, build and run digital solutions that help patients onboard and manage their biologic therapies. We are a team of developers, testers, project managers, behavioural experts and designers who want to help patients.
We like to say that we help patients be better patients by providing them with the tools they need to succeed with their therapies, understand their experience and face new challenges. Our expertise is in working with biologics and drug delivery devices that are used by patients at home.
While our work is predominantly creating connected solutions within the injectables space, I think it’s important to say that a lot of what we do can start in an unconnected world – helping patients onboard with and manage a new medication, even without connectivity. Then, we examine what changes when we add connectivity to that experience and how we can improve on it, but the world doesn’t have to have connectivity to begin with.
“At its core, our work is about understanding patients and empowering them to be successful with their therapies through smart and effective onboarding, training and tracking mechanisms.”
At its core, our work is about understanding patients and empowering them to be successful with their therapies through smart and effective onboarding, training and tracking mechanisms. We look at things from the perspective of patients as people first, rather than focusing on the disease and the drug. We’ve spent well over a decade working in the healthcare space, learning from patients. Sometimes we even go into patients’ homes to understand what’s motivating them to do or not do certain things, so that we can then translate that into digital solutions.
We’ve also learned what’s important for physicians and for brands. And as a result, we are more than just a software developer, we are a strong partner to pharma and device teams as we support them in growing this new area of connected drug delivery.
Q You have a clear passion for the work Rip Road is doing, so what excites you most about the potential of software as a medical device (SaMD) and connected devices more generally?
A Our mission as a company is to impact patients. No one wants to be a patient and no one wants to have to inject medication – it’s not a pleasant experience. So, if we can help patients better understand their experience and make better decisions, that means the world to us.
When we started in this sector, we were working on a rheumatoid arthritis (RA) project where we were helping patients track and understand their RA symptoms. When we interviewed patients to get their feedback, they were so appreciative that their voice was being heard. We helped them see changes in their data so that they could understand and communicate with their doctors better. It was rewarding to shepherd a project through development and see it launch commercially, but the underlying motivation is providing solutions that help patients.
Additionally, while you can use technology to track symptoms without it being SaMD, raising the software to the level where it is a medical device means that we can do more with it as part of the therapy. We can provide real value to the patient, and that’s the goal.
We’ve been involved in digital health for a long time. We’ve worked across disease states to create solutions that engage patients because a more engaged patient has been proven to yield better therapeutic outcomes. Engaging patients at the right time, with the right content and through the right medium is our thing. That experience has informed what we bring to pharma and device clients today. We grew up in a non-pharma world and then applied our learning to pharma, understanding the requirements and guardrails. Today, we are a company for the pharmaceutical space.
Q Continuing from that, let’s discuss your past work. What sort of companies has Rip Road worked with, and what have you learned that has informed your solutions and approaches?
A We’ve worked with a variety of healthcare companies in the past and, today, we primarily work with pharmaceutical teams and drug delivery device teams. It’s no secret that we’ve worked with Amgen for over 10 years, developing a range of SaMD solutions that have been brought to market.
Our experience – working with brand teams, understanding their labels, devices and patient populations – has informed what sort of company we want to be going forwards. It has shaped our software development platform, led us to add our own quality management system and to seek and achieve ISO 13485 certification as we aim to be the gold standard for SaMD and connected devices app work.
“We’ve moved beyond theory and into practice, actually having developed SaMD products that have been brought to market through major pharma partners.”
We’re incredibly proud of the fact that we’ve moved beyond theory and into practice, actually having developed SaMD products that have been brought to market through major pharma partners. It was no small undertaking to work with a pharma client to bring our first connected device app to market a few years ago. We have a real appreciation for that process – the good, the bad, the challenges and things that came naturally. We’ve put serious consideration into questions like “Shouldn’t more of this work happen?” and “How do we help?”. That informs the company we want to be. We want to enable pharma to do more of this work.
One way I put things into context is that I’m a runner – I wear a running watch and look at my data after each run and in the morning after I’ve slept. I’m not looking for a lot of data, just a few key points that, because my watch gives me that information tied to certain things I’m doing, helps me make changes and improve. This is now commonplace on the consumer side of technology, and many aspects of medical devices also already do this. I think it’s natural that it should also happen with drug delivery devices, especially as biologics become more prevalent and more therapies move into the home. We see a role for SaMD solutions and connected devices and ways we can help.
Q Outside of connectivity, especially in the injection device space, the industry has seen a shift towards platform products that use a common basis that is then tuned to the needs of a specific drug product – how important is that principle of building on a proven platform for SaMD products?
A It’s critical. We have a robust, proven platform, which we’re doing good work with. And, because we understand the features and functionalities that help patients with their therapies, as well as the use cases and edge cases, each new assignment gives us new things to learn and add to the platform.
The last thing you want is a monolithic set of code boxing you in. Things are changing rapidly in software development. We offer a platform that allows our clients to iterate and improve solutions that grow with their needs, while working under design controls. It’s been quite a ride to watch technology speed along and then leverage it as a solution developer. What we’re doing in pharma now isn’t old technology; we’re using advanced tools to simplify the patient experience to help them get the most from their therapies.
Q Can you give our readers some more detail about the Rip Road platform itself?
A Broadly, it’s a cloud-based platform for medication management solutions. We’ve prebuilt configurable modules for onboarding, medication management, device connectivity, symptom tracking and more – it’s a long list, which we are always expanding (Figure 1). As a result, our clients can deliver an engaging, branded patient experience in a companion app. Furthermore, we can deploy data portals and dashboards in a broader ecosystem. And of course, we protect and secure all data.
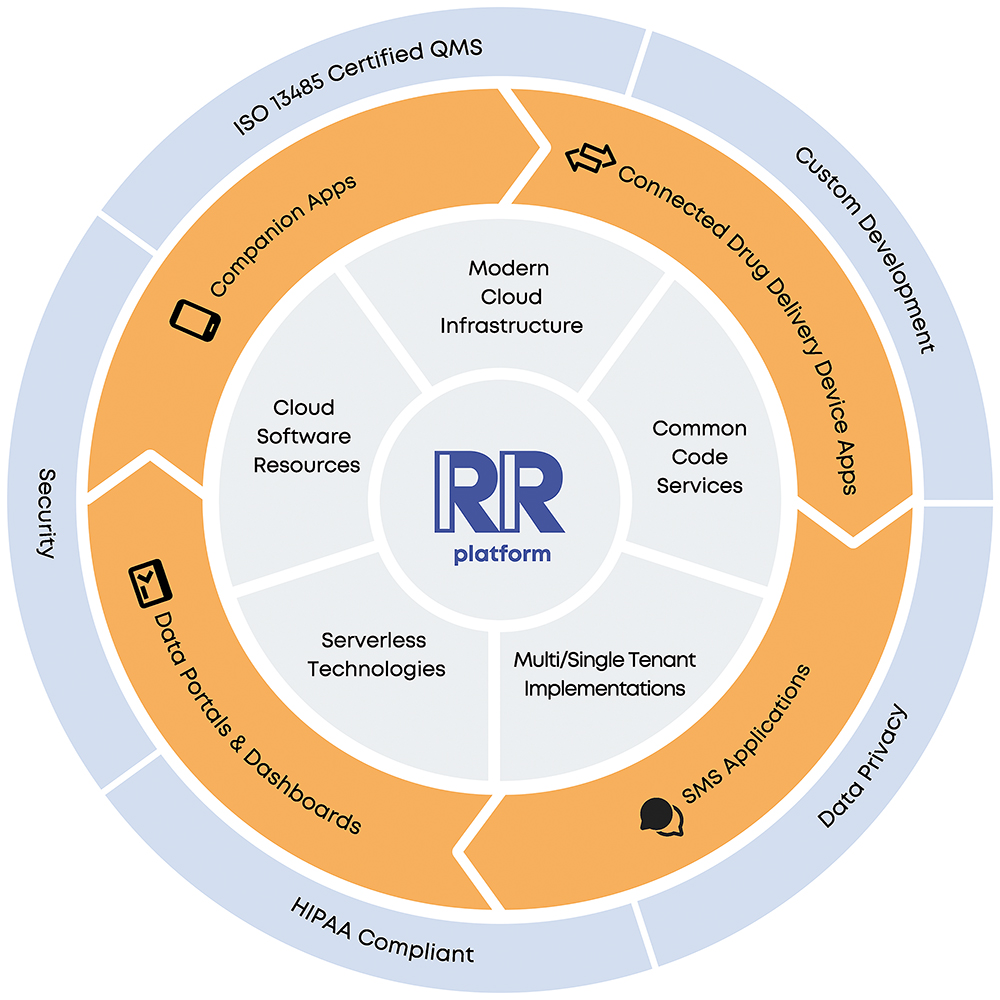
Figure 1: The Rip Road platform.
“One of the things we’ve seen and experienced with our clients is
connectivity used in practice. That’s a really important development.”
I want to highlight our connected device capabilities. We work with a range of medication delivery device types, including autoinjectors, on-body infusion devices, connected accessories and pen caps. We have developed software development kits (SDKs) for device integration and pre-built connectivity guidance and support flows, all to make connecting to new devices easier to set up and easier to manage. Our goal is to lower the barriers to doing device-app connectivity work and enable our clients, both device and pharma, to build solutions, iterate solutions and make changes under design controls as needed.
For example, we have clients on the device side where we’re building digital foundations so that they can sell a full-service offering, device and digital. On the other hand, we have clients on the pharma side where we’re setting up digital ecosystems so that they can learn, test and get into the digital space. We play a role between device and pharma, contributing to both sides. That’s one of the things that’s so exciting for us. There really is a space down the middle where we can help lower the barriers and bring solutions to market.
Q How optimistic are you about the future of SaMD and companion applications? Why should pharma and biologic delivery device companies be excited?
“There’s a greater appreciation on the pharma side than ever before that SaMD exists and can be done. That appreciation wasn’t there five years ago.”
A It goes without saying that I’m excited about the future. We’re currently speaking and working with all parts of the industry, so we see where the interest lies to get this work going. There’s a greater appreciation on the pharma side than ever before that SaMD exists and can be done. That appreciation wasn’t there five years ago. The device side wants it to happen, but we need the business models to catch up, which is where we see an enabling role for Rip Road.
When I look at the industry and see advances on the device side to create better experiences for patients, such as personalised medicine and artificial intelligence (AI) improving R&D and operational aspects, I see SaMD as a complement to that. The device side of the pharma industry has been pursuing connectivity for many years and we’re doing a lot of that work today. The more we all get into it and start learning, the more this work will happen over time.
Q What trends do you see in the industry with regards to connected devices, and what do you think are the ingredients for success with connectivity?
A First and most importantly, device technology is advancing. The second thing I’d mention is that, at some point, there’s going to be an opportunity to feed some of the new AI datasets with actual patient information, but that’s probably a little way out yet.
In the present, one of the things we’ve seen and experienced with our clients is connectivity used in practice. That’s a really important development. Being able to show end-to-end connectivity from a device to an app to a back-end system is crucial for communicating what’s possible with this technology. Often, we’re asked to show a portal view of the data coming in, to which our response is, “Sure, but what do you want to see?” and frequently, the response is, “I don’t know, show me what’s possible.”
That reality had slowed things down because there hadn’t been a clear understanding of what was needed. So, part of the work we’re doing currently is building and demonstrating the technology so that our clients can now touch it and learn about it from hands-on experience. That’s a big deal and a real driver for more of this work happening. Because we’re so involved right now, we’re starting to see opportunities earlier in the development process and areas of potential interest that are worthy of deeper exploration. I’m really excited for that.
Q If our readers were to take away one thing about Rip Road from this interview, what would you want it to be?
A One of the things I like to talk about is how we see ourselves as problem solvers. To do SaMD and connected device work successfully means mastering a wide range of elements – software, quality, risk, devices, connectivity, data and more. There’s a lot to it, such as what data gets shared, why, and how to visualise it. It is hard to do this work well, and my team has this experience; we’ve been in the trenches now for several years, we feel strongly about being good problem-solvers and a good partner to help our clients understand the most important issues and options.
“Despite the complexity of designing companion applications and SaMD for biologics and connected devices, these solutions must be simple to use.”
Despite the complexity of designing companion applications and SaMD for biologics and connected devices, these solutions must be simple to use. They have to be workable for patients and their caregivers. Some patients will need a lot of handholding, others will learn quickly and need less. How do we create solutions that adapt for each patient, are simple to use and provide value? Understanding that complexity and building solutions that meet patients where they are is absolutely critical.
Q Connectivity in the healthcare industry is becoming increasingly important. How has Rip Road contributed to this evolving landscape, and can you share some examples that demonstrate the impact of connectivity on patient care?
A Our area of the industry is helping patents track their medications and providing them with the support tools they need to be successful on therapy. I remember a project a few years ago working with a calcitonin gene-related peptide (CGRP) drug, where our goal was to help patients track their medication injections and the number of migraine days they had in a month, as the labels for the new CGRP drugs all are about minimising migraine days in a calendar month. We built a system for our pharma partner so their patients could track their medication usage and their migraine days, which meant that they could see how much of their month they were getting back, free of migraines, by being on the drug.
My mother was put on a competing drug, and I asked her how she would know if it was working for her. She said, “I think my doctor will tell me.” Meanwhile, we’d already launched a product that allowed patients to have their own data delivered back to them in a way that told them something they didn’t know, empowering them when self-administering their drug. That’s a clear example of exactly what we’re trying to do – help patients have a better experience and make informed decisions when prescribed complex therapies. If we can do just that, we’ll be providing huge value.
To find out more about Rip Road’s SaMD offering for drug delivery devices and biologics, visit: riproad.com.

