To Issue 168
Citation: Isaac T, “Introducing the Terumo Injection Filter Needle – a First Step of the INFINO™ Development Programme”. ONdrugDelivery, Issue 168 (Jan 2025), pp 26–29.
Thomas Isaac considers the issues associated with injection to highly sensitive areas, such as the vitreous body, and the potential to optimise needle design and features to address these issues, discussing the key features of Terumo’s Injection Filter Needle, indicated for hypodermic and intravitreal injections within this context.
Successful drug delivery by injection relies on the selection of a needle well-matched to the application’s requirements, with some therapeutic regimes being more exacting than others. Sensitivity to foreign particulates may be particularly acute in some instances, while higher potential for discomfort and tissue damage is also influential. Improved needle designs and features can offer safer treatment and a better patient experience.
These challenges are exemplified by injection into the vitreous body (eye) for the treatment of increasingly prevalent diseases such as neovascular age-related macular degeneration, diabetic macular oedema, diabetic retinopathy and retinal vein inclusion.1 Estimates suggest that over seven million intravitreal injections are delivered annually in the US alone.2 The scarcity of regulated needles for this application presents an obstacle to market access and means that many of these injections may be performed with disposable needles neither developed nor validated for ophthalmic use, as evidenced from several recent field safety notices warning against off-label use.
REDUCING RISK FOR HIGH-SENSITIVITY INJECTION
Parenteral drug delivery is routine for the treatment of diseases ranging from diabetes to rheumatoid arthritis with daily injection a reality for many. Needle selection plays an important role in defining the patient experience, not only at the point of administration but also with respect to subsequent and long-term complications. The prevention of particulate matter transfer and the need for precise and aseptic delivery are focus points within device development.
The level of visible and sub-visible particles in injectable formulations is a critical quality attribute because, other than the geometry of the needle and the device, there is usually no barrier to prevent their administration to the patient. The associated clinical risks are difficult to fully assess given the range of possible particulates and the difficulty of carrying out robust studies. However, cited complications include phlebitis, granuloma and the obstruction of pulmonary capillaries, the smallest of which are in the region of just 7 μm in diameter.3 For intramuscular and subcutaneous delivery to healthy adult patients, the risks associated with particulate injection are considered to be relatively low but, for immune-compromised patients, those suffering from diseases of the major organs, and neonates and infants, concerns may be considerably higher.3,4 Particulate injection into confined volumes – the eye, a joint or the spine – is also potentially more problematic.3
The drive to minimise the negative clinical impact of injected therapeutics makes particulate contamination an important focus for regulators.5 Standards and test methods for detection are defined in US Pharmacopoeia (USP) <787>, <788> and <789>, but particulate control is challenging. Despite considerable effort, it is likely that millions of particles are injected or infused into patients every day, possibly exacerbating illness and health outcomes.4 Terumo’s literature research shows that the use of filters at the point of delivery may be effective in preventing injected-particulate-related complications, but hypodermic needles with embedded filters are far from common, as noted in USP <789>.
When it comes to other aspects of the injection process, the industry’s understanding of how to improve both safety and the patient experience continues to evolve. For example, the needles used for insulin injection have become progressively shorter and finer – shorter needles help to prevent unintentional intramuscular, as opposed to subcutaneous, injection – while thinner needles are associated with less discomfort, within the constraint of necessarily using larger bores for higher dosage.6,7 Together, the bore and length of the cannula, along with formulation viscosity (given same syringe specification), determine the extrusion force required, with wider bores and shorter cannulas associated with lower injection force.8
Know Your Particulates Particulate contamination is typically classified as either intrinsic, which means that it arises from a material relating to the formulation, its packaging or the manufacturing/assembly process, or extrinsic, which means that it is foreign and unexpected. Glass lamellae resulting from interactions between the formulation and primary packaging are a good example of intrinsic particles; for biologics, there is also the possibility of unexpectedly high levels of protein aggregates. Extrinsic particles might include hair, clothing fibres and paint and are effectively “unknowns”. These, therefore, tend to present the higher risk, especially for aseptic drug delivery.
FOCUSING ON INTRAVITREAL INJECTION
Injection into the vitreous body provides a very specific example of the challenges that can face physician and patient alike with respect to minimising complications, discomfort and tissue damage.
Common issues associated with intravitreal injection include discomfort during the procedure, subconjunctival haemorrhage (a broken blood vessel in the eye), vitreous reflux (the leakage of vitreous humour and drug product) and transiently elevated intraocular pressure.9 In addition, the injection of particulates into the vitreous body, including silicone oil droplets, has been specifically linked with floaters (spots that impair vision), sustained increases in intraocular pressure and endophthalmitis, a rare but severe ocular inflammation that can lead to loss of sight.10 The fact that patients typically require regularly repeated injections to maintain vision increases the likelihood of long-term damage from such complications, focusing attention on equipment and practices that can mitigate risk.
“Reducing discomfort is helpful, not just from the perspective of patient experience but also with respect to sudden eye movement during the procedure and long-term compliance.”
There are frequent references in the literature to the suitability of a 30G needle (or thinner) for intravitreal injection.6,11,12 Wider outer diameter 26G and 27G needles (given the same wall type) have been shown to increase vitreous reflux compared with 29G and 30G needles, as well as being associated with higher levels of discomfort.6 Reducing discomfort is helpful, not just from the perspective of the patient experience but also with respect to sudden eye movement during the procedure and long-term compliance. Both larger and smaller bore cannulas have been linked with lower intraocular pressure, though comparative studies are complicated by the physical properties of the formulation being tested and the dose delivered.13,14
With respect to cannula length, longer needles increase the risk of retinal injury,8,9 as well as the injection and insertion forces required, making them less conducive to gentle, controlled administration. Needles ranging from 8 to 12 mm are routinely referenced, with an upper limit of 18 mm indicated for safe administration.6,8,9
Although prefilled syringes are available for some therapeutics, the process of intravitreal injection typically involves the physician drawing up or transferring the formulation from a vial using a relatively wide bore needle before switching to a finer disposable one for administration. In the absence of needles validated for intravitreal use, standard hypodermic needles are routinely used off-label.15 Ensuring aseptic delivery, protection from the ingress of particulates and precise dosing can therefore be challenging.
INTRODUCING THE INJECTION FILTER NEEDLE BY TERUMO
Terumo has set the specifications of its Injection Filter Needle in collaboration with a leading pharmaceutical company, drawing on extensive in-house experience from past developments (Figure 1). Key features include:
- A needle hub with an embedded polyamide 5 μm mesh filter designed to retain particles in the fluid delivery path
- A 30G 12 mm extra-thin wall cannula (compliant with ISO 15510 and EN 10088-1) – used for Terumo’s K-Pack II needles – that offers a higher flow rate compared with regular wall needles at equivalent injection pressure
- Soft blister packaging to support aseptic presentation and the packaging of drugs in prefilled syringes
- Polymethylmethacrylate (PMMA) hub with threaded flanges – compatible with syringes compliant with ISO 80369-7
- No components made from natural latex.
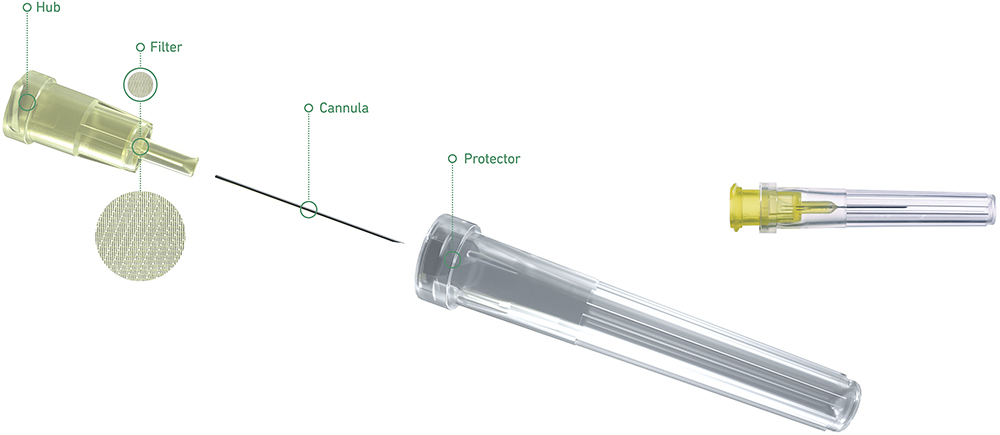
Figure 1: The Injection Filter Needle by Terumo.
“Terumo has carried out the necessary verification and validation processes to support a complete solution for intravitreal injection when combined with a similarly validated syringe.”
These features may help healthcare providers to more effectively protect patients from injected particulates, reduce tissue damage at the injection site, avoid interruptions to the injection process, ensure adequate priming and safeguard aseptic processes. Importantly, Terumo has carried out the necessary verification and validation processes to support a complete solution for intravitreal injection when combined with a similarly validated syringe.
CONCLUSION
Innovations in hypodermic needle design have an important role to play in meeting evolving requirements for drug delivery by injection. With its embedded filter, 30G extra-thin-wall cannula, threaded high transparency hub and blister packaging, the Terumo Injection Filter Needle is an important advancement within this context.
Indication for intravitreal injection is particularly valuable given the scarcity of options for this application and growing clinical need. By working with Terumo to robustly assess the capabilities of the Injection Filter Needle within the context of a target application, product developers and healthcare practitioners can quantify safety and efficacy in use and capitalise on the potential benefits of this new solution.
REFERENCES
- “Retina Health Series: Intravitreal Injections” (Thompson JT, ed). Foundation of the ASRS, 2016.
- Wang R et al, “Quantifying burden of intravitreal injections: questionnaire assessment of life impact of treatment by intravitreal injections (QUALITII)”. BMJ Open Ophthalmol, 2022, Vol 7(1), e001188.
- “Industry perspective on the medical risk of visible particles in injectable drug products”. Parenteral Drug Association, 2014.
- Perez M et al, “Particulate Matter in Injectable Drugs: Evaluation of Risks to Patients”. De Gruyter, June 14, 2016.
- Tawde S, “Particulate Matter in Injectables: Main Cause for Recalls”. J Pharmacovigil, 2014, 3:1.
- Heinemann L et al, “Needle Technology for Insulin Administration: A Century of Innovation”. J Diabetes Sci Technol, 2023, Vol 17(2), pp 449–457.
- “Guidance on choice of needles for Hypodermic insulin devices”. NHS, MKCCG Pharmaceutical Advisers June 2020.
- Feng X et al, “Understanding syringeability and injectability of high molecular weight PEO solution through time-dependent force-distance profiles”. Int J Pharm,2023, Vol 25(631), article 122486.
- Myers L, Almeida D, Abramoff MD, “Intravitreal Injection Technique: A Primer for Ophthalmology Residents and Fellows”. The University of Iowa, Department of Ophthalmology and Visual Sciences, Jan 2015.
- Dounce SM, Laskina O, Goldberg RA, “Particulate Matter from Syringes used for Intravitreal Injection”. Retina, 2021, Vol 41(4), pp 827–833.
- Oztas Z et al, “The short needle intravitreal injection technique”. Int J Ophthalmol, 2016, Vol 9(6), pp 929–930.
- Lam LA et al, “Intravitreal Injection Therapy: Current Techniques and Supplemental Services”. J Vitreoretin Dis, 2021, Vol 5(5), pp 438–447.
- Loureiro M et al, “Intravitreal Injection of Bevacizumab: The Impact of Needle Size in Intraocular Pressure and Pain.’ J Curr Glaucoma Pract, 2017, Vol 11(2), pp 38–41.
- Muto T et al, “Vitreous Reflux Frequency and Intraocular Pressure After First-Time Intravitreal Aflibercept Injections: Comparison of 30- and 32-Gauge Needles”. Clin Ophthalmol, 2020, Vol 14, pp 625–634.
- “Intravitreal Injections”. Facts from the Foundation of the ASRS, Retina Health Series, accessed Jan 2025.

