Citation: Shetty G, Zeiss B, “Microlitre Dosing With Prefillable Syringes – When Does a Device Make Sense?”. ONdrugDelivery Magazine, Issue 97 (May 2019), pp 28-31.
Gautam Shetty and Bernd Zeiss outline factors that need to be weighed when considering a device-based approach to microlitre injections.
Prefilled syringes (PFS) have widely reported benefits in parenteral drug delivery applications from safety, convenience and cost perspectives. It was recently published that in intraocular (intravitreal) injections, the Lucentis® (ranibizumab) prefilled syringe, compared with Lucentis®from a vial, reduced the incidence of infectious endophthalmitis (eye infections) from 0.026% to 0.013% – a halving of cases of an adverse event that can lead to blindness and even death.1
“The drug’s therapeutic window dictates the criticality of accuracy and precision of microlitre drug delivery.”
With the availability of the PFS for Lucentis® and the publicly disclosed impending launch of PFS for Eylea® (aflibercept), PFS are set to become the benchmark for treatments introduced in the ophthalmic space for biosimilars as well as novel therapeutic agents. Lucentis® PFS has also been reported to have a 27-39% reduction in syringe preparation time in one report and 57–63%2 time savings in another; this saving is significant because of the large projected increase in the number of intraocular injections for the treatment of conditions such as age-related macular degeneration (AMD), diabetic macular oedema (DME), vein occlusions and uveitis.
The drug’s therapeutic window dictates criticality of accuracy and precision needed in microlitre delivery. It may seem like the clinical experience of anti-VEGF drugs is evidence proving sufficiency of accuracy with current syringes. A systematic study to check known, related adverse events (for e.g., stroke, cardiovascular events, intraocular pressure, geographic atrophy, etc) relative to accuracy of delivery of anti-VEGF agents is not available. It is yet to be established whether there is a compensatory benefit when presented with increased risk from either underdosing or overdosing of anti-VEGF agents. Besides ophthalmology, there are several other applications that may require microlitre doses – these include dermatology, cell and gene therapy, and vaccines. Many of these applications have a narrower or unknown therapeutic window, requiring better accuracy and precision of the delivered microlitre dose.

Figure 1: Dose reference marking on PFS.
It is also important to delineate the dose volume at which the inherent capability/limitations of a standard plunger stopper prefilled syringe system dictate the need for a device-based approach for microlitre delivery. In this article, we will baseline the capability of the current PFS-only approach to delivering a microlitre dose. For the PFS-plus-device approach to delivering microlitre injections, we will also present important considerations in selecting PFS when combining with a device. The PFS-plus-device approach may be an important consideration for potent drugs for applications in dermatology, oncology and also vaccines, in addition to ophthalmology.
PFS-ONLY APPROACH WITH EXTERNAL DOSE MARK FOR MICROLITRE DOSING
Prefilled Lucentis® makes for an interesting case study as it is indicated at different strengths for AMD (0.5 mg) and DME (0.3 mg). The 0.5mg Lucentis® is a 50 μL dose volume. Rather than deliver a 30 μL dose volume for the 0.3 mg, Lucentis® was reformulated to deliver 0.3 mg in a 50 μL dose. Data has been published showing overlap between a 30 μL and a 50 μL dose using a 1 mL tuberculin syringe (equivalent internal diameter to a 0.5 mL PFS). Thisoverlap encapsulates the challenge where the syringe is operating at the limits of human capability to administer discriminating a sub-50 μL dose from a 50 μL dose. There is data that shows a marked decrease in the confidence of opthalmologists to accurately and precisely deliver a 30 μL or lower dose. Even with 50 μL, ophthalmologists’ confidence is little more than 50%. Yet, the lowest dose volume for a US FDA-cleared ophthalmic drug is 5 μL.
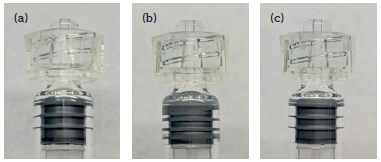
Figure 2: User variation in end-of-injection position of plunger stopper: just bottomed out (a), forced into the shoulder region (b), and retracted having been forced into the shoulder region (c).
The conventional approach would be to reformulate the drug to get the required drug strength to a volume that has precedence of being delivered with a syringe currently in use. Inherent in this approach is the duplication of CMC and related quality assurance efforts to produce, control, maintain and distribute different strengths of the same drug, and hence multiplication of costs. This problem finds particular resonance during clinical trials of drugs dosed in microlitres to study its dose response (evaluation of different strengths of the drug). The additional reformulation effort(s) and other downstream costs constitute a significant portion of clinical trial costs, and add potential risks from having to handle the same drug at different strengths.
Following are some of the factors (unweighted) that have to go right for an accurate, precise microlitre dose to be delivered using a standard PFS-only approach:
- Accurate printing of the dose reference marking (Figure 1)
- No/low variation of syringe internal diameter
- Low break-loose force
- Drug fill amount
- Proper positioning of plunger stopper by user with start of dose reference
- Control by the user of plunger position at the end of injection stroke.
Factors #1 and #2 are controlled and limited by manufacturing capabilities. Marking on the external syringe surface is typically achieved using a pad printing process; the best automated printing process with a custom visual inspection module can yield a dose mark position tolerance of ±0.25 mm . The variation in internal diameter of a PFS is typically ±0.1 mm. However, at additional cost, an internal diameter tolerance of ±0.05 mm can be made available.
| 0.5 mL PFS | 1 mL “long” PFS | |
| Internal diameter variation | ±2.2 μL | ±1.5 μL |
| Dose marking variation | ±4.3 μL | ±8.2 μL |
Table 1 illustrates the expected impact of these variations on the potential inaccuracy of the delivered dose; this is before user-related inaccuracy and imprecision are factored in. Given low fill volumes for applications with microlitre delivery, consideration for factor #3 is necessary to ensure that the user does not accidentally overshoot the target dose mark position when setting the dose. Break-loose force, dead space in the syringe and dead space in the injection needle all need to be factored in to determine an adequate drug fill volume; this is necessary to deliver an accurate, precise microlitre dose.
“The correct choice of PFS is essential relative to the intended application.”
Another source of variation is from the user bottoming out the plunger stopper at the end of the dose (Figure 2). There is clear, visible variation either if the plunger stopper is just bottomed out (a) or if the user forces the plunger stopper into the shoulder region of the syringe (b), hence compressing it. There is variability in this shoulder region as this part is formed in a free-forming process. The variation in dose volume per 0.1 mm of axial travel of the plunger stopper in a 0.5 mL syringe is 1.7 μL and, in the case of a 1 mL “long” syringe, is 3.3 μL. In hypodermic syringes, this overdosing was shown to be as much as 55.2 μL; a >100% dosing error for a 50 μL dose.3 Additionally, should the user remove applied pressure after forcing the plunger stopper into the shoulder region, decompression of the plunger stopper causes the plunger stopper to move back (c). This contributes to additional dose inaccuracy, imprecision and general uncertainty.
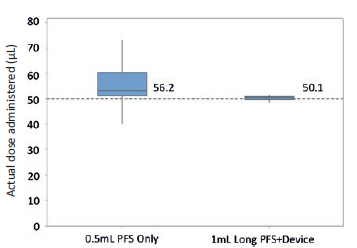
Figure 3: Boxplot with 95% CI of ophthalmologists (n=21) attempting to inject 50 μL with a 0.5mL PFS only and device + 1 mL “long” PFS (n=100) in a lab setting.
PFS-PLUS-DEVICE APPROACH FOR MICROLITRE DOSING
A PFS-plus-device approach to microlitre dosing should certainly be considered when the therapeutic window of the drug is narrow (e.g. potent drugs), irrespective of microlitre dose volume. PFS-plus-device should also be generally considered when the volume to be delivered is less than 50 μL. During clinical development, this consideration extends to the use of hypodermic syringes as well. Devices can either be mechanical or electromechanical. However, electromechanical systems may have limitations in terms of cost, and single-use and secondary sterilisation requirements.In a human factors study, 21 ophthalmologists attempted to inject a 50 μL dose using a 0.5 mL PFS with an external dose mark (Figure 3). The average dose was 56.2 μL (12% error) with
13.2% co-efficient of variation. The lowest injected dose volume recorded was 40 μL and the maximum was 73 μL. This inaccuracy and imprecision could be accommodated for a drug with a large therapeutic window. However, for a drug with a narrow or unknown therapeutic window, allowance of such inaccuracy and imprecision would need to be justified.
A device-based approach for the 50 μL dose volume but with a larger syringe internal diameter (1 mL “long” PFS) showed an average dose of 50.1 μL
(Figure 3) with a 1.6% co-efficient of variation (min volume 48.2 μL, max volume 51.5 μL). The study demonstrated that a PFS-plus-device approach can deliver a more accurate, precise microlitre dose despite a larger internal diameter. The PFS-plus-device approach can be used to attenuate user-related factors causing inaccuracy and provide better control over fill volumes and break-loose force.
This capability can extend to volumes lower than 50 μL, which can provide significant efficiencies and cost benefits when preparing different strength drug solutions during clinical trials. Rather than reformulating and then manufacturing, controlling and distributing different strengths of the same drug, a device can be used to deliver different strengths of the same drug by simply modifying the deliverable dose volume. This approach eliminates several redundancies and helps reduce cost and/or risk.
SINGLE OR MULTIPLE MICROLITRE DOSES
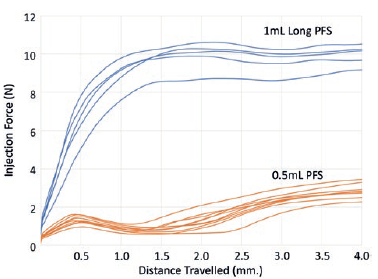
Figure 4: Force with 0.5 mL PFS (orange) and 1 mL “long” PFS (blue), both with baked-on silicone when injecting water with 30-gauge ½” needle.
Published studies have shown that there is a tendency towards overdosing when it comes to delivering microlitre injections. However, if more than one microlitre dose is to be administered using a single syringe, it is expected that overdosing would be followed by eventual underdosing. Such dose-fractioning applications require a PFS-plus-device approach to simplify the injection procedure, and avoid bifurcation of the clinician’s cognitive bandwidth between dose measurement and anatomical considerations of the injection site.
PFS CONSIDERATIONS IRRESPECTIVE OF PFS-PLUS-DEVICE OR PFS-ONLY APPROACHES
Injection force: Ophthalmic and dermatological applications require injection using a fine needle (30 gauge or finer). This has an impact on the injection force. As shown in Figure 4, injection force with a 0.5 mL PFS is lower than a 1 mL “long” PFS. This difference may bemore acute for viscous formulations. The device could provide mechanical advantage to help reduce the injection force even when accuracy of dose is not an issue. Lower injection force for stability is useful when delivering an injection in applications such as ophthalmology and dermatology.
Luer lock adapter removal torque: Luer lock is the most appropriate PFS configuration to be used for most microlitre applications. The Luer lock adapter on a glass PFS is typically a plastic component press-fit onto the glass Luer cone, having a tiny undercut. In human factors studies, it was observed that none of the clinicians held the Luer lock collar when attaching the needle. It is hence important to ensure that torque to dislodge/rotate the Luer lock adapter (Figure 5) is always greater than either torque for the user to attach the syringe or torque for the user to remove the tip cap.
Subvisible particulates: Applications such as ophthalmology require conformance with USP<789> for subvisible particulates. Baked-on silicone or silicone-free PFS can help confirm to this requirement.
Other requirements: Other PFS requirements for microlitre applications may include low endotoxin levels, silicone free, tungsten free, low oxygen and moisture permeability, and allowance of drug storage as low as -80°C.
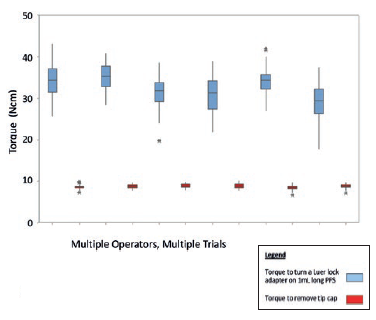
Figure 5: Torque to turn a Luer lock adapter on 1 mL long PFS compared with torque to remove tip-cap, with 95% confidence intervals for multiple operators and trials.
CONCLUSION
When does a PFS-plus-device approach make sense? A PFS-plus-device approach is essential when accuracy and precision is necessary in the delivery of a microlitre dose. A PFS-plus-device approach is also required for doses of 50 μL or less for drugs with a narrow or unknown therapeutic window. Irrespective of whether a PFS-plus-device or PFS-only approach is employed for microlitre dosing, the correct choice of PFS is essential relative to the intended application.
REFERENCES
- Storey P et al, “The Impact of Prefilled Syringes on Endophthalmitis Following Intravitreal Injection of Ranibizumab”. Am J Ophthalmol, 2019, Vol 199, pp 200-208.
- Subhi Y et al, “Prefilled syringes for intravitreal injection reduce preparation time”. Dan Med J,2016, 63(4).
- Song M et al, “Volume evaluation of additional anti-VEGF agents released when the plunger is double pressed”, Association for Research in Vision & Ophthalmology (ARVO) 2019 Annual Meeting, Vancouver, Canada, April 28-May 2, 2019, Poster A0294.
BIBLIOGRAPHY
- Gerding H et al, “Accuracy and precision of intravitreallyinjected ranibizumab doses: an experimental study”. Klin Monatsbl Augenheilkd, 2010, Vol 227, pp 269-272.
- Busimi A, Shetty G, “Drug Injections Into The Eye: Unmet Clinical & Compliance Needs, Opportunities For Novel Microliter Dosing PFS”. Presentation at 2014 PDA Universe Of Prefilled Syringes and Injection Devices Conference, Huntington Beach, CA, US, October 6-7, 2014.
- Gerresheimer internal technical data for 1 mL long TELC syringe with baked-on silicone.
- Congruence Medical Solutions internal technical data for microlitre dosing syringe device system.
- Meyer C et al, “Accuracy, precision and repeatability in preparing the intravitreal dose with 1.0cc syringe”, Acta Ophthal, 2012, Vol 90(2), e165.

