To Issue 160
Citation: Scannell P, “Navigating the Transition from MDD to MDR for Co-Packaged Combination Products”. ONdrugDelivery, Issue 160 (May 2024), pp 53–56.
Paul Scannell explains how to navigate the European regulatory landscape for co-packaged drug-device combination products safely.
“Combination products, by their very nature, are interdisciplinary products and, consequently, from a regulatory perspective, can present various layers of complexity.”
In purely mathematical terms, the consequence of combining two elements together is a simple exercise in arithmetic. In this scenario, the whole can be viewed as the aggregate of its parts. However, in the right circumstances, additional value can be realised when combining the right elements and deliberately maximising the synergy between the distinct parts based on an understanding of their resulting interaction.
One example of such holistic synergy is combination products. Combination products consist of two or more regulated products (i.e. a medicinal product, a medical device or a biological product) which, when brought together, can result in an enhanced whole – one that delivers a more effective, more efficient or more accessible treatment pathway for patients.
These benefits are reflected in the fact that a quarter of approved medicines in the EU include a medical device component, providing an indication of the increasingly influential role of combination products within modern healthcare systems and their increasing importance to patients.1 These products have established this position thanks to ongoing advancements in drug delivery technologies expanding device capabilities and because of underlying shifts in healthcare trends. For example, the drive to enable greater levels of self-care and for treatments to be delivered in non-clinic environments, including the home, can only be made possible if patients or caregivers have access to both the required medicine and an accessible, simple method of administration.
However, combining a medical device and a medicinal product is not a simple arithmetic exercise. Combination products, by their very nature, are interdisciplinary products and, consequently, from a regulatory perspective, can present various layers of complexity. The European regulatory environment for combination products, which is markedly different from the system in the US, necessitates an intimate understanding of two separate, yet closely linked, regulatory frameworks that must be navigated in all their complexity to successfully bring a combination product through development and onto the European market (Figure 1). Specifically, these regulatory frameworks are the European Medical Device Regulation (MDR), Regulation (EU) 2017/745 and the Medicinal Products Directive (MPD), 2001/83/EC.
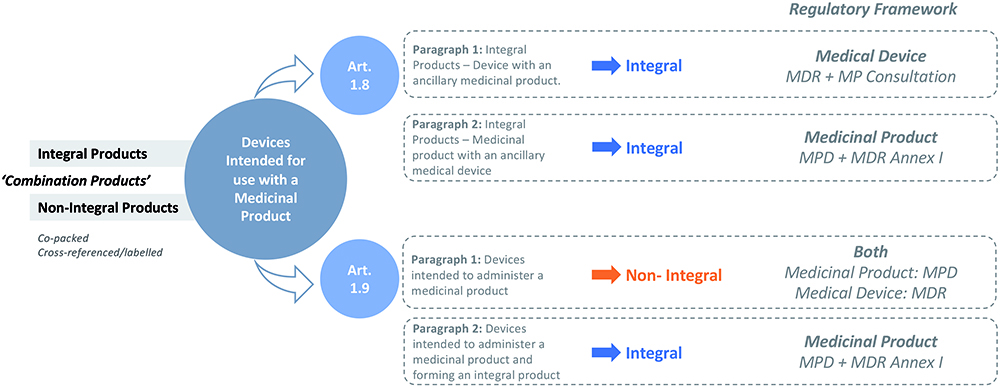
Figure 1: Planning drug product packaging testing with drug product development can help reduce delays in regulatory submission.
“The associated regulatory challenges are particularly pronounced in the case of medical devices subject to a first CE marking in parallel with the medicinal product marketing authorisation.”
A NEW REGULATORY FRAMEWORK FOR THE EU
The MDR is a new regulatory framework, which became fully applicable from May 26, 2021, replacing the Medical Device Directive (MDD) 93/42/EEC. The goal of the MDR was to establish and ensure a robust, transparent and sustainable regulatory framework, and to maintain a high level of safety while also supporting innovation. As with the introduction of any new piece of legislation, challenges will inherently be encountered in implementation, and this holds true in the case of the MDR.2 This is evidenced by the extension of the transition period for medical devices previously certified in accordance with the MDD to be re-certified in accordance with the MDR.3 While this deadline has shifted for some device types, reflecting the challenges in conforming to this updated regulatory framework, the unintended consequences and regulatory uncertainty introduced by the MDR have important implications for owners of co-packaged combination products.
The regulatory process for co-packaged combination products may initially appear straightforward. Simply put, the medicinal product requires marketing authorisation alongside evidence that the co-packaged device is compliant with the entirety of the MDR (i.e. CE-marked) before the combined product is placed on the market.4 However, therein lies the challenge for co-packaged combination products: they face a duality of predominantly independent approval processes. The EU does not have an integrated approach in the evaluation, as well as the lifecycle management, of such co-packaged combination products, and lacks comprehensive coordination between the medicinal product and medical device regulatory frameworks.
The associated regulatory challenges are particularly pronounced in the case of medical devices subject to a first CE marking in parallel with the medicinal product marketing authorisation. For what is effectively placed on the market as one combined product, two separate applications must be made to two distinct and disconnected regulatory bodies under two approval timeframes that are not co-ordinated. The thresholds for co-ordinating successful applications between the medicinal product manufacturer and the medical device manufacturer can therefore be seen as higher, while the potential for unintended points of conflict and the risks impacting approval success are increased.
ENSURING DEVICES COMPLY WITH THE MDR
These risks can be mitigated by using a medical device that has already received its CE certification. The medicinal product manufacturer must, however, ensure that the intended use of the medical device mirrors the intended use when co-packaged with the medicinal product. Furthermore, the medicinal product manufacturer should be cognisant of the status of the medical device’s CE mark as it is their responsibility, when submitting a MAA, to ensure that the medical device(s) co-packaged with the medicinal product is in compliance with the MDR (or the MDD if applicable) before the combined product is placed on the market.4 However, where the device has been CE marked in accordance with the MDD and is availing of the MDR transitional provisions, the marketing authorisation holder (MAH) should inform itself of the device manufacturer’s transition plan to CE marking under the MDR to ensure that the device can continue to be placed on the market once the applicable transition timeframe has expired.
Where the intended device to be co-packaged has not yet been CE marked, the co-packaged device must comply with the MDR, and the challenges that exist for standalone medical devices (i.e. devices placed on the market in their own right) are equally applicable.2 These challenges are further compounded for co-packaged combination products under the dual regulatory framework and are exposed early on in development.
ADDRESSING THE DUAL REQUIREMENTS OF SEPARATE REGULATORY FRAMEWORKS
When it comes to premarket clinical studies for co-packaged combination products, for example, no current process exists to allow for an integrated and streamlined regulatory submission and approval. The medical device and medicinal product are, again, subject to two separate regulatory frameworks governing clinical studies: the MDR and the Clinical Trial Regulation (CTR) EU No 536/2014 respectively.
“When it comes to product approval assessments, the parallel requirements for medicinal products and medical devices can result in duplication of reviews and inconsistencies in feedback from regulatory bodies.”
Similar to the CTR, the MDR also provides for a co-ordinated assessment procedure for clinical investigations of medical devices in line with Article 78. However, in lieu of a fully functional EUDAMED, no co-ordinated procedure is currently available, thereby necessitating individual authorisation by each member state in which the clinical investigation is to be conducted. The lack of a co-ordinated assessment for a medical device therefore negates the efficiencies brought about by the CTR in the case of premarket co-packaged combination product clinical studies. In acknowledgement of this disconnect, for co-packaged and other types of combined studies, the European Commission has signalled its intent to analyse the root causes of the challenges encountered by sponsors in conducting combined studies and to identify possible solutions to these challenges through the COMBINE project.5
While solutions are being sought in this area, challenges remain in others. When it comes to product approval assessments (i.e. medicinal product marketing authorisation and medical device conformity assessment), the parallel requirements for medicinal products and medical devices can result in duplication of reviews and inconsistencies in feedback from regulatory bodies. Applicants could be left with potentially contradictory strands of information relating to, for example, clinical evaluations and risk assessments, all of which might be understood when applied to a device in isolation but do not offer a definitive, coherent perspective on a combination product.
One specific area that often results in inconsistent regulatory obligations is the regulatory requirements for labelling, which must also be met independently for both device and medicinal product, despite the obvious need for them to be consistent and complementary. Separation of these processes can introduce the risk of confusion or contradiction, underlining the need to maintain a unified approach.
Furthermore, there is no defined duration for medical device conformity assessment leading to CE marking. This contrasts with the structured approval timescales for medicinal products, resulting in a potential misalignment that can trigger unpredictable complications or delays to product launches.
AREAS OF CONCERN – FROM SUPPLY CHAIN TO SCIENTIFIC ADVICE
Following launch, challenges with the dual frameworks for medical device and medicinal product regulations do not go away. One example is the potential for pharmaceutical companies to be positioned in the role of an economic operator under the MDR (i.e. distributor or importer), further to the requirement to adhere to the good distribution practice guidelines for the pharmaceutical supply chain. This relates to the fact that, despite a co-packaged drug-device combination being registered as a medicinal product – implying that regulations for medicinal products should take precedence – the co-dominant requirements of the MDR also apply and necessitate an intimate understanding of the supply chain, not just of the co-packaged product but also of the medical device part.
In addition, there is no integration between the pharmacovigilance and vigilance systems for each of the respective elements, resulting in a two-pronged approach for what is effectively a single product. Furthermore, any subsequent variations to the product must be managed in the absence of a well-defined and clearly integrated post-market change engagement pathway, with potential for complication and delays.
Another area where there is a divergence between the two regulatory systems is in scientific advice. The aim of medicinal product scientific advice, at any stage of a medicine’s development, is to provide prospective regulatory and scientific guidelines to help developers on the most appropriate path to generate robust evidence on a medicine’s benefits and risks so that no major objections regarding the design of the tests are likely to be raised during the evaluation of the MAA.6 Notified bodies, however, are precluded from providing similar advice during the development of a medical device. Pre-submission dialogues are therefore essential to set the level of device evidence expectations and would improve the quality and correctness of submissions for conformity assessment. Efforts are being made in this area through the EMA in the form of a scientific advice pilot for high-risk medical devices regarding clinical development strategies and clinical investigation proposals.7 However, more concerted and co-ordinated efforts are required to help address some of the unique and broader challenges faced by developers (both medicinal product and medical device) of combination products.
PARTNER INSIGHT IS KEY TO COMPLIANCE FOR CO-PACKAGED PRODUCTS
While there are initial attempts at co-ordinating a dual regulatory framework for co-packaged combination products, including the proposed reform to the pharmaceutical legislation,8 it is crucial to understand the subtle details of this specific regulatory environment to facilitate the regulatory journey, identifying potential problems ahead of time and guaranteeing successful market access.
As a leading provider of devices that are frequently co-packaged with medicines to create effective non-integral combination products, West Pharmaceutical Services’ regulatory team is experienced in overcoming specific challenges that may occur due to unintended consequences of the MDR on co-packaged combination products, working with pharma partners to provide valuable insights into how to effectively address any issues.
In terms of process, insights from device partners can help to improve planning efficiency. They can bring attention to areas that are likely to encounter challenges or delays and ensure that crucial results are prioritised from the beginning. Furthermore, a partnership approach allows information requests to be managed concurrently and in parallel, facilitating responses that are consistent at an overarching product level and avoiding late-stage iterations.
THE BENEFITS OF A CO-ORDINATED AND COLLABORATIVE APPROACH
In an ideal world, the need to navigate such complex matters would not be so demanding and the regulatory framework affecting co-packaged combination devices would be more unified, as it is in the US. Indeed, West is among the industry voices advocating for the EU to improve the regulatory framework for co-packaged combination products while retaining absolute focus on effectiveness and patient safety. As it stands, both new and existing co-packaged combination products destined for the European market continue to be burdened with a fragmented and opaque regulatory framework.
“If devices are integral to positive patient outcomes, then device partners must play an integral role to ensure regulatory compliance is achieved in the most effective way.”
The impact of this situation is felt not only by applicants but also potentially by patients through time-to-market delays, aborted submissions or even withdrawal of products from the market – all of which can erode momentum behind bigger picture ambitions for innovative, cost-efficient treatments that can empower patients to take greater control of their own care and ease pressures on healthcare systems.
To mitigate these risks and adapt to the changing needs of the industry, it is crucial that the owners of co-packaged combination products consider collaboration as a primary strategy. Put simply, if devices are integral to positive patient outcomes, then device partners must play an integral role to ensure regulatory compliance is achieved in the most effective way. They can be seen as a catalyst and navigational aid, streamlining the process and optimising the time, cost and effort involved in tandem with pharmaceutical partners – a unified, integrated approach that undoubtedly delivers more than the sum of its parts.
REFERENCES
- “Regulatory Road To Innovation”. Report, EFPIA, Mar 2022.
- “The Future of Europe’s Medical Technology Regulations”. Web Page, MedTech Europe, accessed Apr 2024.
- “Regulation (EU) 2023/607 of the European Parliament and of the Council of 15 March 2023 amending Regulations (EU) 2017/745 and (EU) 2017/746 as regards the transitional provisions for certain medical devices and in vitro diagnostic medical devices”. European Parliament, Mar 2023.
- “Questions & Answers for applicants, marketing authorisation holders of medicinal products and notified bodies with respect to the implementation of the Medical Devices and In Vitro Diagnostic Medical Devices Regulations ((EU) 2017/745 and (EU) 2017/746)”. EMA, Nov 2023.
- “Combined Studies”. Web Page, EC, accessed Apr 2024.
- “Scientific advice and protocol assistance”. Web Page, EMA, accessed Apr 2024.
- “EMA pilots scientific advice for certain high-risk medical devices”. Press Release, EMA, Feb 27, 2023.
- “Reform of the EU pharmaceutical legislation”. Web Page, EC, accessed April 2024.

