To Issue 152
Citation: Metzmann F, “Needlestick Injury Risks: Safeguarding Healthcare Workers and Patients”. ONdrugDelivery, Issue 152 (Oct 2023), pp 70–74.
Fred Metzmann discusses how using safety syringes can be a cost-effective measure to reduce the risk of injury to healthcare personnel and patients, and introduces Roncadelle Operations‘ devices designed for safer drug administration.
Needlestick injuries (NSIs) are a risk to healthcare workers and patients that can lead to dangerous and potentially life-threatening infections, including hepatitis B, hepatitis C and HIV. In EU countries alone, more than one million cases occur annually.1 In the UK, compensation for NSIs between 2012 and 2017 cost the NHS more than £4 million.2 The economic burden of NSIs in Italy, for example, is estimated at €72 million (£61 million), only including direct costs for diagnostics, prophylaxis and post-exposure monitoring.3
“NSIs and sharps injuries represent the most common occupational injuries among healthcare workers, with an incidence of approximately 41%.”
In many EU countries, despite the so-called “Sharps Directive” (Directive 2010/32/EU), there is still suboptimal adoption of safety syringes. A survey by HOSPEEM (European Hospital and the Healthcare Employers’ Association) and the EPSU (European Federation of Public Services Union) investigated the concerns associated with poor compliance with the Directive. Among them, they noted a lack of adequate resources, training programmes and effective data collection systems.
It is essential that institutions and healthcare organisations facilitate consistent interpretation and widespread implementation of the Directive, as well as the ISO 23908 standard, which defines the safety requirements for the design and manufacture of devices to ensure compliance with the EU regulation.
Currently, there are medical devices available on the market that guarantee full compliance with the quality and safety standards established by the Directive. Roncadelle Operations’ syringes are simple to use, require a minimal amount of force to activate and include an automatic safety mechanism to avoid any potentially harmful actions by the operator.
AN UNDERESTIMATED HEALTH PROBLEM
While approximately 50% of injuries go unreported, in Italy alone, around 100,000 percutaneous exposures occur each year.4 NSIs and sharps injuries represent the most common occupational injuries among healthcare workers, with an incidence of approximately 41% (Figure 1).5
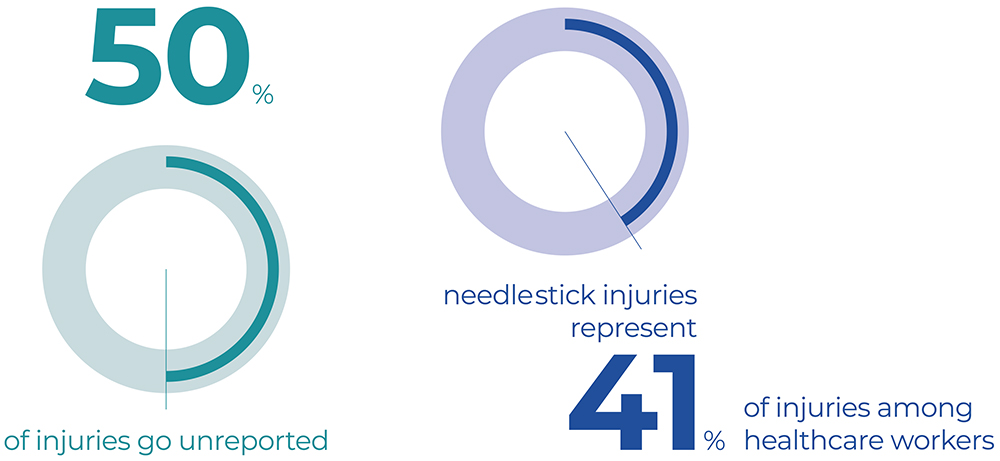
Figure 1: Sharps and NSIs represent the most common occupational injuries among healthcare workers, accounting for approximately 41% of all injuries.
Accidental NSIs are the most common and can occur during a wide range of procedures, including blood sampling, suctioning, drug administration, inserting catheters and handling clinical waste. These injuries occur most commonly among nurses, and the physical and emotional impact of such incidents can be severe and long-lasting.
The economic burden of NSIs, only considering the direct healthcare costs for diagnostics, prophylaxis and post-exposure monitoring, is estimated at about €850 per event,3 and this is excluding any indirect costs incurred due to loss of productivity and workers’ compensation.
“Over the last decade, the prevention of sharps injuries has become a high priority topic across the EU, which has led to the adoption of the Directive 2010/32/EU10 for the prevention of sharps injuries in the hospital and healthcare sector.”
A SERIOUS BUT AVOIDABLE RISK
NSIs primarily result from the misuse of safety syringes, which incorporate protective mechanisms that, upon activation, create a lasting barrier between the hands and the needle until proper disposal.
Studies conducted in the EU have demonstrated that the use of safety syringes with integrated protection mechanisms, together with training programmes to educate staff appropriately and the improvement of working conditions, could prevent 80% of NSIs (Figure 2).6,7 Such measures would significantly reduce healthcare costs and stress, enhance workers’ productivity and improve the patient’s experience.
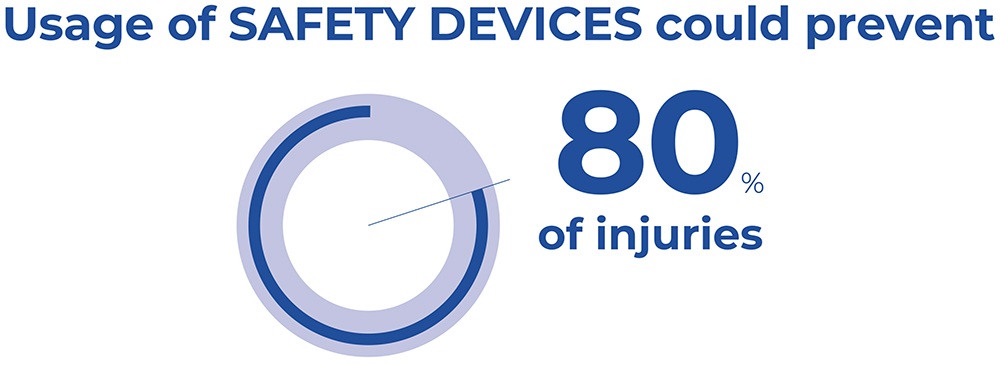
Figure 2: Safety devices could prevent 80% of injuries.
In the current healthcare situation – characterised by continuous growth in pharmaceutical innovation alongside an increase in chronic conditions, such as diabetes, cardiovascular diseases and autoimmune diseases – it is important to consider the growing role of prefilled safety syringes for subcutaneous administration. The relevance of these devices relates to the use of new drugs that require special dosing accuracy and are frequently administered or self-administered at the patient’s home. One study estimated that by 2027, the global value of the prefilled syringe (PFS) market will surge from its current $5.9 billion to about $9 billion at an annual growth rate of 9%.8
THE REGULATORY FRAMEWORK
Over the last decade, the prevention of sharps injuries has become a high priority topic across the EU, which has led to the adoption of the Directive 2010/32/EU10 for the prevention of sharps injuries in the hospital and healthcare sector. The aim is to provide “the safest possible working environment through the prevention of injuries caused by all types of medical sharps devices.”
The Directive introduced the framework Agreement negotiated by the sector’s European social partners HOSPEEM and EPSU. For example, in Italy, the Directive was incorporated through Legislative Decree No. 19 of February 19, 2014,9 which amended Legislative Decree No. 81/2008 to enhance health and safety in the workplace. In Germany, the EU Directive 2010/32/EU on protection against sharp injuries in the health sector is implemented through the TRBA 250. This guideline mandates employer responsibilities in Section 4.2.5, building on the BioStoffV’s regulation for mandatory safety devices. The regulatory framework specifies the minimum requirements that must be adopted by the member states to protect workers.
The requirements include:
- Risk assessment – mapping of all work areas and situations where the potential for injuries exists
- Adoption of appropriate preventive measures – with particular attention to the use of medical devices with integrated safety and protective mechanisms, and the elimination of unsafe devices
- Reporting in case of accidents and injuries – currently, 50% of cases are unreported
- Information and training programmes to raise awareness and provide practical instruction on the correct use of devices with integrated protective mechanisms.
“Promoting a safe working environment is a priority for modern healthcare systems, despite a shortage of doctors and nurses in Europe.”
CRITICAL ISSUES AND POSSIBLE AREAS FOR ACTION
In February 2017, HOSPEEM and the EPSU adopted a joint work programme for the period 2017–2019 with the aim of monitoring the implementation of the Directive in the EU member states and, above all, implementing the measures stipulated in the legislation.10
A survey, directed to HOSPEEM and EPSU national affiliates,11 was conducted to examine the areas where adoption of the Directive has produced positive benefits in preventing sharps injuries and to identify the existing challenges with the implementation of the legislation in order to identify ameliorative solutions that could be be applied in the different national contexts.
The suboptimal adoption of safety-engineered protection mechanism devices, which mainly affects Southern European countries (Italy, Spain and Greece), was among the most critical issues that emerged from the survey. A lack of sufficient financial resources, with the consequence that many hospitals are still buying equipment without safety mechanisms, thereby disregarding the regulatory provision, was cited as one of the main problems by respondents from Spain and Norway. In these countries, the need for hospitals to restrict spending puts facilities at risk of incurring much higher costs for the management of occupational incidents.
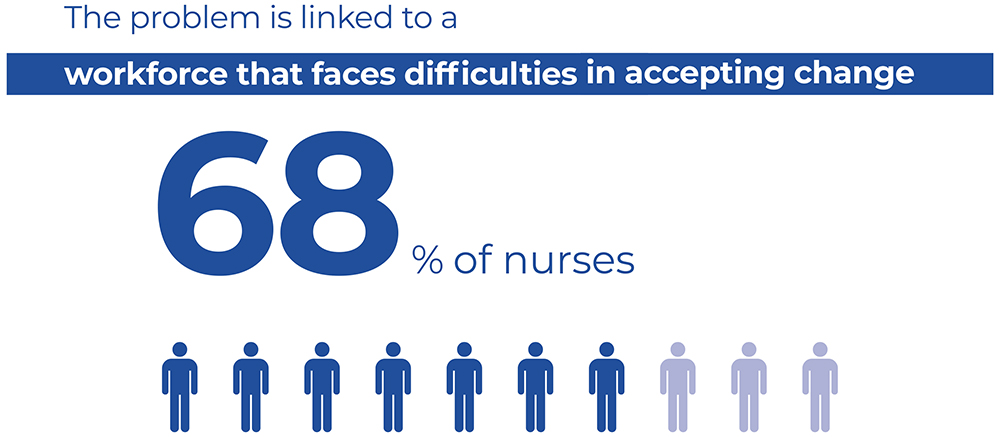
Figure 3: There is difficulty amongst healthcare workers when it comes to adopting new, innovative devices, as reported by 68% of Italian nurses.
A second challenge highlighted in the survey relates to difficulty within the workforce of adopting new, more innovative devices, as reported by 68% of Italian nurses (Figure 3). Another issue is the non-comprehensive coverage of training programmes by all categories of staff who are potentially at risk. In particular, this relates to temporary workers, such as trainees, students and interns, as highlighted by respondents from Spain and Austria.
The lack of an effective data collection system for percutaneous exposure incidents at the national level does not facilitate the identification and rectification of the causes and corresponding corrective measures. The issue of underestimating the number of cases due to the non-reporting of sharps injuries emerged as particularly relevant in Italy, Spain, Germany and Norway.
The working group has drafted recommendations aimed at protecting healthcare personnel and patients from the risk of injury, thereby preventing the transmission of dangerous infectious diseases:
- The allocation of adequate resources for the purchase of quality medical devices equipped with safety-engineered protection mechanisms as part of a broader strategy aimed at eliminating occupational risk, which has a net positive impact on healthcare facilities’ budgets
- The adoption of common procedures for the procurement of medical devices and materials
- Revision of the national regulation on reporting percutaneous exposures with the aim of reducing the number of unreported injuries and facilitating the sharing of best practices at the European level
- Funding for training activities for healthcare workers that focuses on the use of the latest generation of medical devices.
SAFETY SYRINGES: A SAFE AND EFFECTIVE WAY TO REDUCE NSIs
The WHO recommends the use of safety-engineered syringes with a reuse prevention feature to eliminate or reduce the risk of infection as much as possible for workers and patients.12
Promoting a safe working environment is a priority for modern healthcare systems, despite a shortage of doctors and nurses in Europe. EN ISO 23908:2013 outlines the requirements and test methods for evaluating the performance parameters of sharps injury protection features for medical devices containing hypodermic needles for single use, introducers for catheters and lancets, and other needles used in blood sampling.
As part of the EC’s proposal for standardisation of the Medical Devices Regulation 2017/745 (MDR) and the In Vitro Diagnostic Medical Devices (IVDM) Regulation 2017/746, EN ISO 23908:2013 is listed among the “existing harmonised standards to be revised” by May 27, 2024, concerning “technical solutions for safety mechanisms to be applied in the design and manufacture of devices to ensure compliance with Sections 11.1 and 22.2 of Chapter II of Annex I of Regulation (EU) 2017/745. The standard applies to devices intended to be used for the administration and/or extraction of body/blood fluids and/or medicinal substances.”
Given the importance of addressing the issue of NSIs, it is imperative that there should be no gaps in the interpretation of the MDR regarding general safety and performance requirements. This is crucial for manufacturers and to avoid disparities in protecting the safety of healthcare workers, patients and the community. The key elements are that safety syringes should be as simple as possible to use, require a minimal amount of force for activation and should include an automatic safety mechanism to avoid any potentially dangerous intervention from the user.
Highlighting the real risk of uneven implementation of the regulation, the Spanish General Nurses Council and European Biosafety Network have published an Interpretation Guide aimed at ensuring consistency in the implementation and interpretation of the MDR about the requirements defined in Annex 1, Sections 11.1 and 22.2.13
TECHNOLOGICAL INNOVATION FOR THE PREVENTION OF NSIs
There are medical devices already on the market that guarantee full compliance with the quality and safety standards established by the regulations. Roncadelle Operations has been developing minimally invasive and safe devices for the administration and self-administration of drugs for more than two decades, making use of state-of-the-art technologies and production facilities.
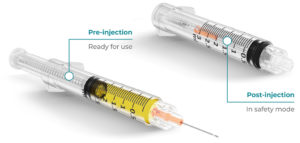
Figure 4: Roncadelle SafeR® syringe.
SafeR® Syringe
The SafeR® passive safety syringe with retractable needle has several safety features that ensure accurate dosing of the drug, allow the needle to automatically retract at the end of the injection, avoid contact between the operator and the patient and prevent reuse of the device (Figure 4).14
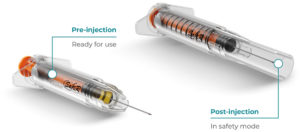
Figure 5: Roncadelle SafeR® Shield for Prefilled Syringes.
SafeR® Shield for Prefilled Syringes
Similarly, in the SafeR® Shield PFS, the needle is fully covered by the shielding system on completion of the injection. Additionally, as the drug does not come into contact with anything but the syringe, there is no need for drug stability studies (Figure 5).14
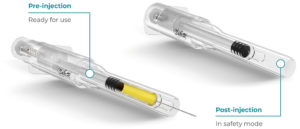
Figure 6: Roncadelle SafeR® Reverse for Prefilled Syringes.
SafeR® Reverse for Prefilled Syringes
In the SafeR® Reverse PFS, the shielding system automatically activates after the injection, and the needle is fully covered. The design eliminates the need for stability studies as the drug does not come into contact with anything but the syringe (Figure 6).14
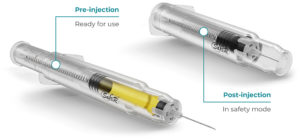
Figure 7: Roncadelle SafeR® Car-GO for Cartridges.
SafeR® Car-GO for Cartridges
In the SafeR® Car-GO syringe, the entire cartridge and needle retract on completion of the injection. As the drug only touches the cartridge until the injection, the syringe does not require drug stability studies (Figure 7).14
CONCLUSIONS
To minimise the risk of NSIs and protect healthcare workers and patients from potentially serious infections, the following recommendations are desirable. Firstly, the consistent interpretation and universal implementation of the Medical Sharps Directive 2010/32/EU and the Medical Devices Regulation (EU) 2017/745, as well as the updated ISO 23908 standard (by May 2024). Secondly, the rigorous implementation of syringes that adhere to the highest safety standards, specifically those with passive safety mechanisms and automatic needle retraction, is essential. It is equally crucial to phase out and eliminate devices that do not meet these safety benchmarks. Furthermore, the introduction of nationwide surveillance to guarantee the purchase and use of safety devices, monitor accidental exposures and collect up-to-date data on NSIs.
Find out more at: www.roncadelle-operations.com.
REFERENCES
- “Proposal for a Council Directive implementing the Framework Agreement on prevention from sharp injuries in the hospital and healthcare sector concluded by HOSPEEM and EPSU”. European Commission, October 26, 2009.
- “Did you know? Preventing needlestick injuries”. NHS, accessed Aug 2023.
- Cazzaniga S et al, “Il costo delle ferite accidentali da aghi e l’impatto dei dispositivi di sicurezza per la prevenzione dal rischio di punture accidentali”. Mecosan, Italian Quarterly of Health Care Management, Economics and Policy, N. 58, 2006.
- Puro V et al, “Aggiornamenti in tema di epidemiologia delle malattie infettive occupazionali trasmesse per via ematica”. G Ital Med Lav Erg, 2010, Vol 32(3), pp 235–239.
- Mohammed A et al, “Global prevalence of needle stick injuries among nurses: A comprehensive systematic review and meta-analysis”. J Clin Nurs, 2023, Vol 32(17–18), pp 5619–5631.
- Ottino MC et al, “Needlestick prevention devices: data from hospital surveillance in Piedmont, Italy – comprehensive analysis on needlestick injuries between healthcare workers after the introduction of safety devices”. BMJ Open 2019;9:e030576.
- Tosini W et al, “Needlestick injury rates according to different types of safety-engineered devices: results of a French multicenter study”. Infect Control Hosp Epidemiol, 2010, Vol 31(4), pp 402–407.
- “Prefilled Syringes Market Size, Trends and Growth Analysis by Material (Glass Syringes, Oil Siliconized Syringes, Baked on Silicone Syringes and Plastic Syringes), Type (Conventional Prefilled Syringes and Safety Prefilled Syringes), Design (Single-Chamber Prefilled Syringes, Dual-Chamber Prefilled Syringes and Customized Prefilled Syringes), End User (Hospitals/Clinics and Ambulatory Surgical Center) and Region (Americas, Europe, Asia-Pacific and Middle East & Africa) – Forecast till 2032”. Research Report, Market Research Future, Aug 2019.
- “Prevenzione delle ferite da taglio o da punta nel settore ospedaliero e sanitaria”. Altalex, Legislative Decree, 19/02/2014 No. 19, O.J. 10/03/2014, accessed Aug 2023.
- “Follow-up on the Directive 2010/32/EU on the prevention from sharps injuries in the hospital and healthcare sector”. HOSPEEM – EPSU report. 2019, accessed Aug 2023.
- “HOSPEEM-EPSU Survey Assessment Implementation Sharps Injuries Directive 2010/32/EU & Problems & Action Needed”. European Public Service Union, Dec 15, 2017.
- “WHO guideline on the use of safety-engineered syringes for intramuscular, intradermal and subcutaneous injections in health care settings”. WHO, accessed Aug 2023.
- “Interpretation guidance on annex i, points 11.1 and 22.2 of EU regulation 2017/745 of the European parliament and of the council”. European Biosafety Network, 2021, accessed Aug 2023.
- “Solutions”. Company Web Page, Roncadelle, accessed Aug 2023.

