Citation: “Product Showcase: Injay – Connected Prefilled Syringes” . ONdrugDelivery Magazine, Issue 103 (Dec 2019), pp 38-39.
The need for connected solutions is growing and all healthcare players are committed to providing solutions that make patients’ lives easier and improve compliance. However, until now, there has been no solution to answer the need to connect a prefilled syringe (PFS).
More than five billion PFSs are used each year, and the self-injection market is constantly growing. PFSs are widely used to administer vaccines, anticoagulants, anti-arthritic, anti-anaemic and many other drugs. And the biologics approval rate is intensifying, with a wide variety of new products being cleared by the US FDA and the EU EMA. Most biologics are delivered through injections, making them attractive to use with a PFS, and there is a growing demand for home therapies and easy adoption of devices.

Figure 1: Injay, the connected prefilled syringe.
There are four fundamental principles that a connected PFS must respect: treatment adherence of commercialised drugs, quality of reporting, secure treatment protocol and traceability. With Injay (Figure 1), the first prefilled syringe with built-in connectivity, Biocorp provides a simple, cost-effective and easy-to-implement solution. The use of Injay is totally transparent and does not interfere in any way with the user’s traditional injection process.
Injay is a built-in solution for PFSs, based on a custom finger flange (with backstop function) and a custom syringe piston rod with a near-field communication (NFC) label. The device is adaptable to any standard size of PFS (0.5, 1, 2.25 mL etc) or material (glass, plastic/cyclo olefin polymer etc). Injay is able to track any state of injection completion and collects key information such as time and date, drug type, batch number and expiry date. Moreover, the data can be customised up to 150 characters so that any appropriate information can be encoded. The shape and colour of the backstop can also be customised, to meet patients’ needs better, and to maintain ergonomic and intuitive handling of the device.
The information is sent via NFC technology to an app on a smartphone or tablet. Recent updates on commercially available smartphone terminals now enable coverage and reading of NFC technology on iOS and Android platforms. However, the choice of the reader can be adapted according to the drug and healthcare setting – special NFC readers could be provided if the injection is delivered in hospital,for example, or could even be integrated in a sharps container so as not to require any additional effort from the patient or healthcare practitioner.
Injay is a cost-effective solution since it is easy to integrate into the conventional industrialisation process. The finger flange and custom piston rod with NFC chip are assembled after the conventional filling process of the PFS. Injay has many fields of application (Figure 2), as the device is adaptable to different contexts and environments.
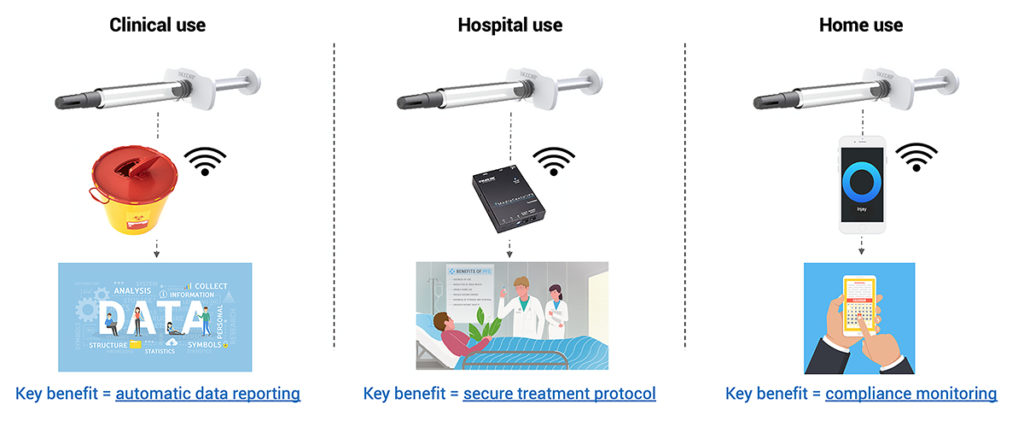
Figure 2: Overview of potential applications of Injay, in clinical trials, in the hospital setting and at home.
It could be a suitable solution for connecting autoinjectors, for example, where a case-by-case integration assessment will have to be made. It can optimise clinical trial performance – key information is automatically collected and transferred to a mobile application, allowing data to be archived and subsequently accessed and analysed. It can also help to check the degree of adherence and compliance – answering the question of whether a patient has correctly followed the prescriber’s recommendations. The device also guards against reporting errors.
Injay can be used by patients at home – offering compliance monitoring as it reports that the injection is made in its entirety, and also reporting the time and date.
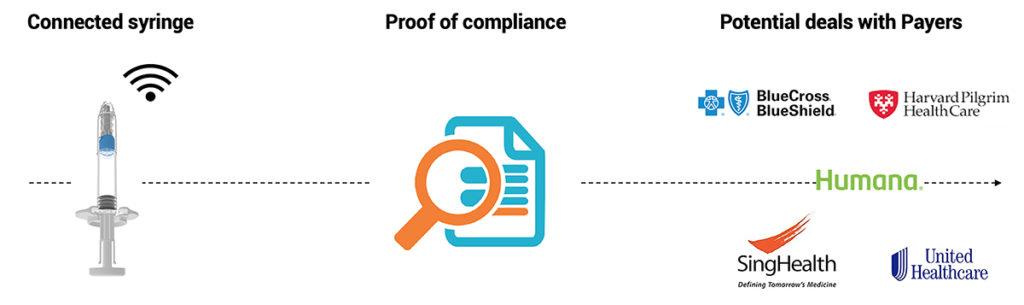
Figure 3: Injay presents a clear path towards better reimbursement or market protection.
At a time of cost constraints in major markets, Injay enables pharma companies to provide a simple and reliable solution to payers for tracking the correct use of a drug and could facilitate negotiations on price-to-performance tracking strategies (Figure 3). Injay is due to be available on the market for clinical testing early in 2020.

