Citation: Frearson T, “Pushing the Boundaries of Infusion Pump Interoperability”. ONdrugDelivery Magazine, Issue 98 (Jun 2019), pp 68-71.
Interoperability is not a new concept for infusion pumps. But in many cases hospitals have been slow to embrace the full potential of connectivity. When is this likely to change? What will hospitals need when it does? And where should infusion pump manufacturers focus innovation efforts in the meantime? Tim Frearson considers the options.
It’s more than 15 years since smart infusion pumps were first introduced. Wireless connectivity offered exciting new opportunities to improve the management of pump populations and integrate them with the wider healthcare system. Instead of operating in isolation, multiple pumps could be joined up with a single server, enabling software revisions to be uploaded remotely. And monitoring several patients’ infusion status from a single station without having to visit the bedside was a tantalising prospect.
These were just some of the promised benefits, and it was expected that they would quickly translate into better efficiency and treatment. Yet just because the technical capabilities exist, it doesn’t necessarily mean they are embraced. Unfortunately, parachuting new technology into healthcare environments rarely delivers tangible improvements unless the workflow around it also changes. So a modern infusion pump with a digital maintenance log might be returned to the technical team with a handwritten label that simply says “broken”. And, most of the time, nurses still dash to the bedside to differentiate between critical, non-critical and false alarms when infusion pumps need attention.
AN ONGOING JOURNEY
“Unfortunately, parachuting new technology into healthcare environments rarely delivers tangible improvements unless the workflow around it also changes.”
In 2014, Tim Vanderveen (now retired but then Vice-President of the CareFusion Center for Safety and Clinical Excellence in San Diego, CA, US, since acquired by BD), described a future vision for infusing patients safely:1
- All infusion pumps will be connected to a hospital wireless network
- All infusion pumps will be associated with a specific patient and clinician
- Image recognition (barcode, radio frequency identification) will be used to identify the intravenous (IV) drug/concentration being infused
- Infusion pumps will be automatically programmed from the pharmacist-reviewed physician’s order
- Smart pump safety software will protect against programming errors during titrations, bolus dosing and STAT (i.e. urgent) infusions, and in operating room procedure areas
- All IV infusion data will be automatically recorded in each patient’s electronic medical record in near-real time
- Infusion status of all infusions will be available to pharmacy to facilitate IV compounding
- Discrepancies between in-process infusions and computerised prescriber-order-entry orders will be identified for resolution
- Critical lab values and missing lab values that impact IV infusions will be immediately communicated to the appropriate caregiver
- All patients receiving high-alert IV medications will be continuously monitored with appropriate vital sign monitoring, even outside the ICU
- Infusion pump alarms and alerts will be sent directly to the appropriate caregiver(s)
- Infusion pump performance issues will be automatically communicated to biomedical staff.
Five years later, many of these points are still futuristic for most hospitals.
Vanderveen’s paper is quick to point out that interoperability is a journey, not a destination. This is true of all transformative developments in the digital economy, and the sheer scale of healthcare organisations – coupled with the importance of patient safety – makes it difficult to introduce changes at pace.
However, demands to deliver better patient outcomes whilst improving healthcare efficiency continue to escalate. This means at some point the journey will accelerate. As healthcare systems become more digitally enabled, infusion pumps could represent an effective starting point for more sophisticated and integrated closed-loop systems.
As Vanderveen said: “Several factors make infusion pumps an ideal starting point for interoperability: the very large number of devices in a typical hospital, the critical importance of the drugs being infused, the growing base of wirelessly connected pumps already installed and their uniquely bidirectional information transfer, both to and from the pump.”1
He added that development could manifest itself in various ways, depending on individual hospitals’ priorities. One might choose to focus initially on the ability to send alarms directly to the clinical team. Another might consider interfacing smart pumps with barcode medication administration systems. The ultimate goal would be a fully integrated, closed-loop medication system – but this would most likely be achieved gradually over time, with small advancements driving cumulative benefits.
WHEN WILL INTEROPERABILITY TAKE OFF?
There are signs that the healthcare sector could be about to reach a tipping point in terms of digital transformation. Deloitte estimates that the Internet of Medical Things market will be worth more than US$158 billion (£121 billion) in 2022.2 Infusion pumps will certainly form part of this. According to MarketsandMarkets, the global infusion pump market is expected to reach almost $16 billion by 2023, up from $12 billion in 2018.3
In this environment, infusion pump manufacturers have many opportunities to develop their product offering. But the million-dollar question is how and where to focus innovation. Is it possible to take device capabilities to a higher level, so they play a more sophisticated role in connected drug delivery? Or would it be better to evolve technologies for easier integration with existing systems? Could closed-loop systems offer new ways to overcome enduring infusion pump challenges, like those associated with air-in-line false alarms? What about addressing variations between different markets and different healthcare segments? After all, infusion pump challenges and demands in an intensive care unit (ICU) setting are quite different from those on a general ward. And there is stark variation in requirements between developing countries and the most developed nations.
It’s difficult to know where to start, but you must start somewhere and, when you consider how long it takes for an infusion pump to transition from concept to active operation, time is of the essence. Many businesses and entrepreneurs will be looking to earn a slice of this potentially lucrative market. Unless action is taken, well-established infusion pump manufacturers could suddenly find themselves at a disadvantage, with disruptive new entrants cornering emerging opportunities.
BARRIERS TO INTEROPERABILITY
“Well-established infusion pump manufacturers could suddenly find themselves at a disadvantage, with disruptive new entrants cornering emerging opportunities.”
Various practical and cultural challenges hinder existing smart infusion pumps from achieving more. Finding ways to overcome these could help trigger widespread uptake of interoperability more quickly.
Regional variations
At present, US hospital systems tend to be more progressive than the rest of the world. They have the advantage of large-scale institutions and installations underpinned by multi-million-dollar investments. Other regions, including Europe, are less advanced. The UK’s NHS is a case in point. Limited funding, combined with an ageing hospital infrastructure and a diverse mix of devices connecting to hospital systems, means the environment is fraught with problems. Interoperable devices sometimes require ad hoc interfaces to support their integration with the wider system and ensure they deliver value. In less developed countries such as Romania and Hungary, caregivers are well-educated and keen to benefit from the improved performance, accuracy and safety associated with the latest devices. However, they lack the infrastructure to exploit capabilities fully.
Practical matters
Interoperable infusion pumps can face conflicting demands, depending on the department where they’re deployed.On a general ward, patients are typically connected to one or two infusion pumps. But in an ICU, an individual patient might receive drugs and nutrients from 20 or more devices, so space around the patient bedside becomes a significant consideration.
In some regions, infusion pumps need to be compatible with docking stations. These enable multiple devices to be located efficiently around the bedside, so they don’t get in the way of the clinical care team. However, an individual pump may also have to work as a standalone device and operate independently from the docking station – for example, if the patient is to be transferred to a general ward. Furthermore, when multiple smart pumps try to connect to Wi-Fi simultaneously, it poses software architecture challenges. Overcoming technical issues like these could improve usability and act as a stepping stone for infusion pumps to unlock fully interoperable healthcare.
Outside the US, there is still an extensive need for standalone infusion pumps. They may have Wi-Fi capability installed but it’s not necessarily used to begin with. Since the typical life of a service pump is seven years, the rationale behind this approach is sound. Within that timeframe, infrastructures may evolve, meaning devices need to be futureproofed so connectivity can be leveraged later. We explored device connectivity trends in detail in a 2018 article for ONdrugDelivery.4 Developing infusion pumps with scalable capabilities – so they can easily adapt to healthcare environments as they change – could be a prudent move for manufacturers.
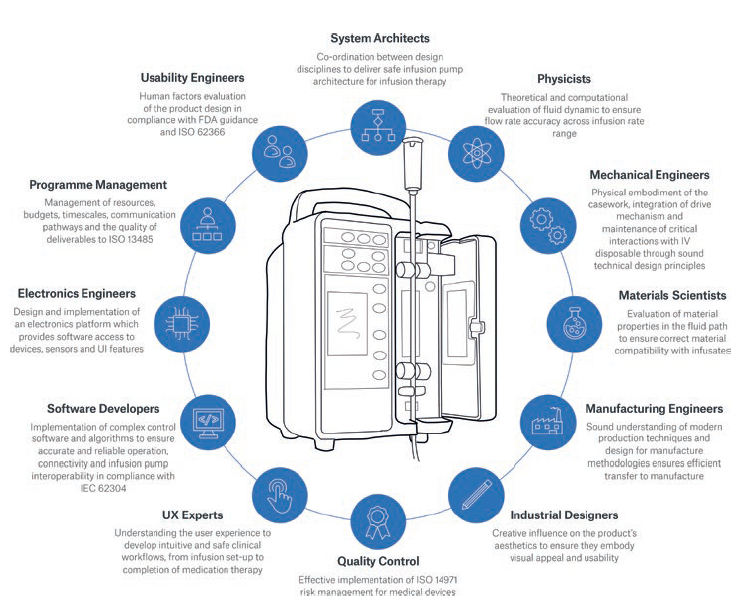
Figure 1: The multidisciplinary skills involved in innovative infusion pump design and development.
Cybersecurity
Cybersecurity issues are a vital consideration for all smart medical devices. The US National Cybersecurity Center of Excellence (NCCoE) and National Institute of Standards and Technology (NIST) have flagged specific risk factors related to connected infusion pump ecosystems:
“With an increasing number of infusion pumps connecting to networks, the vulnerabilities and risk factors become more critical, as they can expose the pump ecosystem to external attacks, compromises, or interference.”5
This has led the NCCoE to develop an “example implementation”. It demonstrates how healthcare delivery organisations can use standards-based, commercially available cybersecurity technologies to protect the infusion pump ecosystem, including patient information and drug library dosing limits.5 Infusion pump manufacturers must address both patient safety and security when developing smart products. Software architecture decisions made early in the process have a significant bearing on this.
MULTIDISCIPLINARY INNOVATION
“Drawing on a wide range of skills can reveal new opportunities for innovation that might otherwise be overlooked.”
Overcoming challenges and maximising opportunities for infusion pumps in the digital age demands a wide range of specialist expertise. Figure 1 shows the extent of multidisciplinary input required, and the role each element plays.
Historically, infusion pumps have had long development lifecycles led by mechanical, electrical and software engineering disciplines. But to achieve the closed-loop interoperability vision, further emphasis on the wider system is required. This means systems architects, software developers and human factors experts have an increasingly important role to play. Developing architectures that can cope with multiple devices competing for Wi-Fi bandwidth is a complex challenge which has the potential to directly impact patient safety. Cybersecurity is also a serious concern. Manufacturers need to draw on the skills of experienced systems engineers and architects who can make effective and informed decisions in this rapidly evolving environment.
Input from physicists, materials scientists, and simulation and analysis experts is also required at an early stage in the innovation process. Their expertise in matters such as fluid dynamics (to ensure efficient mechanism characteristics across wide flow-rate ranges, whilst maintaining fluid-flow accuracy) and material properties (to ensure compatibility with infusion medication) plays a critical role. Having all this expertise available under one roof ultimately accelerates the product development process and timescales.
Drawing on a wide range of skills can reveal new opportunities for innovation that might otherwise be overlooked. Take the air-in-line issue. It’s vital that infusion pumps alert medical staff if there is a risk of a large air bubble entering a patient’s bloodstream. However, sensors can be triggered unnecessarily on a frequent basis, which creates the potential for nuisance alarms and can be disturbing for patients.
The combined knowledge of materials scientists, chemists and physicists could identify which aspects of pumps, their consumables and infusion medication exacerbate the false-alarm problem.For example, the fluidic properties of some drugs mean they are prone to forming harmless microbubbles on the inside surface of the IV line that may “bounce” in the location of the air-in-line sensor due to the action of the pump mechanism. Also, some IV tube materials suffer from the “cold weld” phenomenon where the tube takes on a permanent set after being in use for a period of time. This can result in the IV tube geometry decoupling from the air-in-line sensor so that air pockets form between the outside of the tube and the sensor.
Both the above scenarios can result in the detection of air and subsequent false alarms – but collaborative multidisciplinary innovation might eradicate such problems. Overlaying developments like this with elements of Vanderveen’s future vision for infusing patients safely in a closed-loop system could truly revolutionise healthcare outcomes and efficiency, one step at a time.
Co-ordinating this depth and breadth of technical input in a cohesive way is no mean feat, but setting robust design controls at the outset of an innovation project helps. It ensures complex needs and requirements are addressed properly and in a timely fashion. When barriers and potential problems are identified at the outset, their avoidance or elimination becomes central to the strategy. However, if they emerge towards the end of the process, they can stall a project in its final stages. This can lead to significant financial repercussions, or even prevent FDA market approval.
CONCLUSION
It’s fair to say that the needle hasn’t moved significantly since Vanderveen set out his future vision for infusion pumps in 2014. But, to quote Bill Gates: “We always overestimate the change that will occur in the next two years and underestimate the change that will occur in the next ten. Don’t let yourself be lulled into inaction.”6 The Internet of Medical Things is gathering momentum, and healthcare systems will transform – albeit at a slower pace than other sectors. To remain relevant as this unfolds, infusion pump manufacturers need to develop a multidisciplinary mindset that supports and accelerates innovation.
REFERENCES
- Vanderveen T, “From Smart Pumps to Intelligent Infusion Systems –The Promise of Interoperability”. Patient Safety & Quality Healthcare, May 27, 2014.
- Ronte H, Taylor K, Haughey J, “Medtech and the Internet of Medical Things: How connected medical devices are transforming health care”. Research Report, Deloitte Centre for Health Solutions, July 2018.
- “Infusion pumps market worth 15.89 billion USD by 2023”. MarketsandMarkets, April 2018.
- Harvey C, “Normalising Connectivity: Could Unconnected Devices Become the Exception?”.ONdrugDelivery Magazine, Issue 87 (Jun 2018), pp 56-59.
- O’Brien G et al, “Securing Wireless Infusion Pumps in Healthcare Delivery Organizations”. Special Publication, US National Institute of Standards and Technology, August 2018.
- Gates B, “The Road Ahead”. Book (published by Viking Penguin), November 1995.

