To Issue 170
Citation: Dantas C, Krueger U, Burnett H, “Realistic Breathing Profiles in In Silico Models: a Partnership Approach”. ONdrugDelivery, Issue 170 (Apr 2025), pp 16–21.
Carolina Dantas, Ulf Krueger and Howard Burnett discuss the critical need for precision when delivering biologics to the lungs, how PULMOTREE’s Kolibri is able to achieve this using guided breathing, and how the use of realistic breathing profiles can improve outcomes and decrease risk with in vitro and in silico testing.
THE CHALLENGES OF INHALED BIOLOGICS
Respiratory delivery of biologics presents significant challenges, particularly when it comes to ensuring efficient and reproducible drug deposition in the targeted lung regions. A device that can provide precise drug delivery is crucial for maximising therapeutic effects while minimising drug losses, particularly given the high cost of biologics, their complex formulations and the need to maintain stability throughout the delivery process.
One of the primary factors that influences the efficacy of pulmonary drug delivery is the patient’s breathing manoeuvre, which can vary significantly. Variability in breathing patterns can lead to inconsistent lung deposition, impacting both therapy efficacy and safety. Studies have shown that, if the breathing manoeuvre is controlled, this variability can be reduced.1 As demand for more efficient drug-nebuliser combinations grows, innovative solutions that enhance aerosol delivery and improve targeted lung deposition are essential.

Figure 1: Kolibri™ Mesh Nebuliser platform.
“KOLIBRI USES A HAPTIC FEEDBACK MECHANISM TO SUPPORT AND GUIDE THE PATIENT’S INHALATION, ENSURING AN OPTIMAL BREATHING PATTERN THROUGHOUT THE NEBULISED TREATMENT.”
THE KOLIBRI™ MESH NEBULISER: ADDRESSING THE CHALLENGES
The Kolibri Mesh Nebuliser platform addresses these challenges with its guided inhalation system, integrating an innovative feedback mechanism that optimises aerosol performance, minimises variability in patient breathing patterns and ensures precise drug deposition (Figure 1). Kolibri uses a haptic feedback mechanism to support and guide the patient’s inhalation, ensuring an optimal breathing pattern throughout the nebulised treatment. This haptic feedback works by guiding users to maintain an ideal inhaled flow rate, providing a pleasant positive vibration when inhalation remains within a predefined optimal range. Conversely, if the inhalation flow rate exceeds this limit, the system provides a discouraging negative vibration to prompt adjustment.
To further reduce aerosol loss, in addition to its breath-triggered mechanism, Kolibri only activates nebulisation when the patient’s inhaled flow rate stays within a predefined optimal range tailored to the target deposition area. The device’s Intelligent Administration System (iNAS) technology promotes extended inhalations, guiding users to sustain each breath for up to the individual’s maximum capacity to enhance the inhaled volume and therefore obtain more deep lung drug deposition.
Additionally, Kolibri automatically measures and records detailed parameters regarding the breathing manoeuvre, such as inhaled flow rate, duration and adherence, thereby enabling the creation of a comprehensive characterisation of the patient’s breathing profile on an inhalation-by-inhalation basis. These real-time monitoring capabilities provide valuable insights into adherence and therapy effectiveness.
FEEDBACK TECHNOLOGY SUCCESSFULLY REDUCES PATIENT VARIABILITY
PULMOTREE has conducted detailed validation testing to assess the effectiveness of Kolibri’s feedback technology. The study recorded and analysed volunteers’ breathing profiles while inhaling saline solution with Kolibri. The results demonstrated that the feedback system can successfully guide users’ breathing manoeuvres by encouraging deeper and slower inhalations, defined as an inhaled flow rate below 15 L/min for six seconds, with high consistency.2 A relevant reduction of both intra- and inter-patient variability associated with individual breathing manoeuvres was observed in most of the participants (Figure 2).
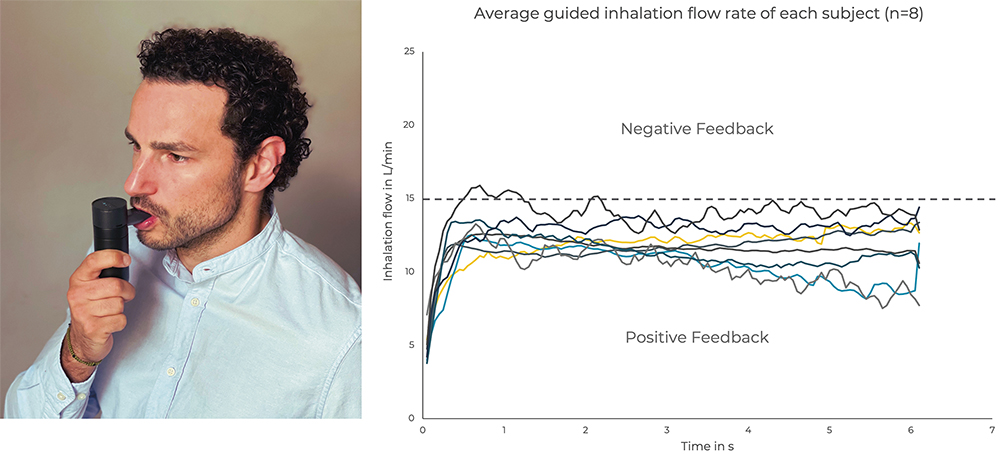
Figure 2: Participant (A) of the Kolibri™ device validation study and the reduced inter-individual variability of the average inhalation flow rate (B).
“THIS STUDY RESULTED IN THE ESTABLISHMENT OF A GUIDED BREATHING PROFILE FOR KOLIBRI, WHICH IS REPRESENTATIVE OF HOW PATIENTS USE THE DEVICE.”
This study resulted in the establishment of a guided breathing profile for Kolibri, which is representative of how patients use the device. This realistic breathing profile is based on user data from the validation test, where the feedback technology was set to guide a “long and deep inhalation” (Figure 3), and the profile parameters include an inhaled volume of 1500 mL, an inhalation time of six seconds, an inhalation-to-exhalation ratio of 1.6:6 and a peak flow of 15 L/min.
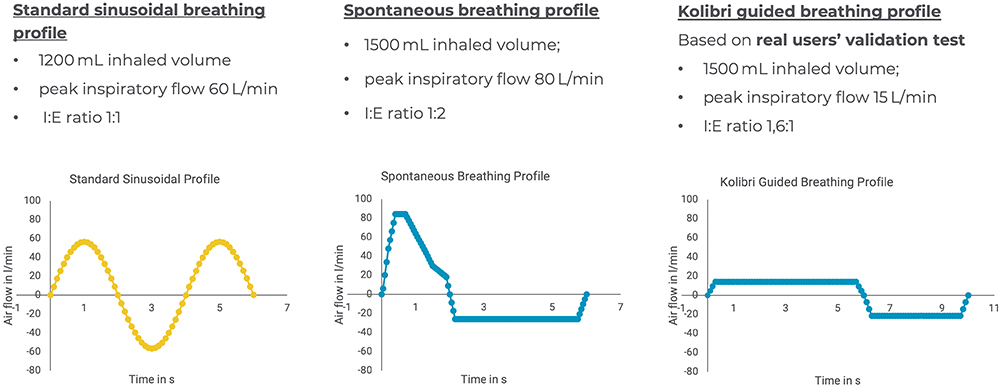
Figure 3: Comparison of standard sinusoidal (yellow) versus realistic breathing profiles (blue), spontaneous unguided and Kolibri guided, applied to investigate the differences in regional lung deposition.
THE POTENTIAL OF REALISTIC BREATHING PROFILES IN AEROSOL CHARACTERISATION
Current in vitro testing standards, such as USP <1601> and Ph Eur 2.9.44, rely on standardised breathing profiles that fail to reflect real patient use, often leading to discrepancies with in vivo outcomes. Incorporating realistic breathing profiles into in vitro and in silico testing can enhance the predictive accuracy of aerosol drug delivery systems by addressing these limitations.
- In Vitro Testing: Using realistic breathing profiles in delivered dose testing can provide more representative and predictive results than standard sinusoidal patterns.
- In Silico Modelling: Integrating patient-specific breathing profiles and lung geometries can enable more precise simulations of regional lung deposition, optimising drug formulation and device matching.
By incorporating realistic inhalation data during early development phases, pharmaceutical companies can improve the predictive power of their studies, ensuring a better match with real-world performance. This approach helps to mitigate risks in drug-device development, reducing costly late-stage failures and streamlining regulatory approval processes.
A COLLABORATIVE CASE STUDY: OPTIMISING AEROSOL DEPOSITION
A collaboration between PULMOTREE and Aptar Pharma through Nanopharm, Fluidda (Kontich, Belgium) and Ockham Biotech (Fareham, UK) showcases how current joint capabilities can enhance drug-nebuliser performance.
OCK4 by Ockham Biotech is a heparin derivative, classified as a high molecular weight biologic, developed to be nebulised and target the small airways and alveolar lung regions. To ensure optimal drug delivery, the Kolibri Mesh Nebuliser platform was tailored specifically for OCK4 through an appropriate mesh selection and customisation of individual device features.
“IN THE CONTEXT OF THE KOLIBRI-OCK4 COMBINATION PRODUCT DEVELOPMENT, PULMOTREE AIMED TO ASSESS THE IMPACT OF THE KOLIBRI GUIDED BREATHING PROFILE ON THE DEPOSITION OF OCK4 THROUGH ADVANCED IN SILICO SIMULATIONS.”
In the context of the Kolibri-OCK4 combination product development, PULMOTREE aimed to assess the impact of the Kolibri guided breathing profile on the deposition of OCK4 through advanced in silico simulations. Using two different in silico models, a comparison was made between the regional lung deposition of OCK4 using two different breathing profiles – a realistic Kolibri guided breathing profile versus a spontaneous unguided breathing profile (Figure 3).3
Regional Deposition Model
A model based on the National Council on the Radiation Protection and Measurements (NCRP) model implemented in the Mimetikos Preludium software (Emmace, Lund, Sweden) was developed by Nanopharm to predict aerosol distribution in an idealised healthy lung geometry (Weibel model of the lung). The Kolibri guided breathing profile demonstrated (Figure 4):
- 45% higher deposition in the target regions for OCK4 (bronchiolar and alveolar) compared with the spontaneous breathing profile
- A more favourable pattern for deep lung deposition due to higher alveolar deposition (41.3% versus 27.9%) and lower extra-thoracic deposition (12.4% versus 23.4%).
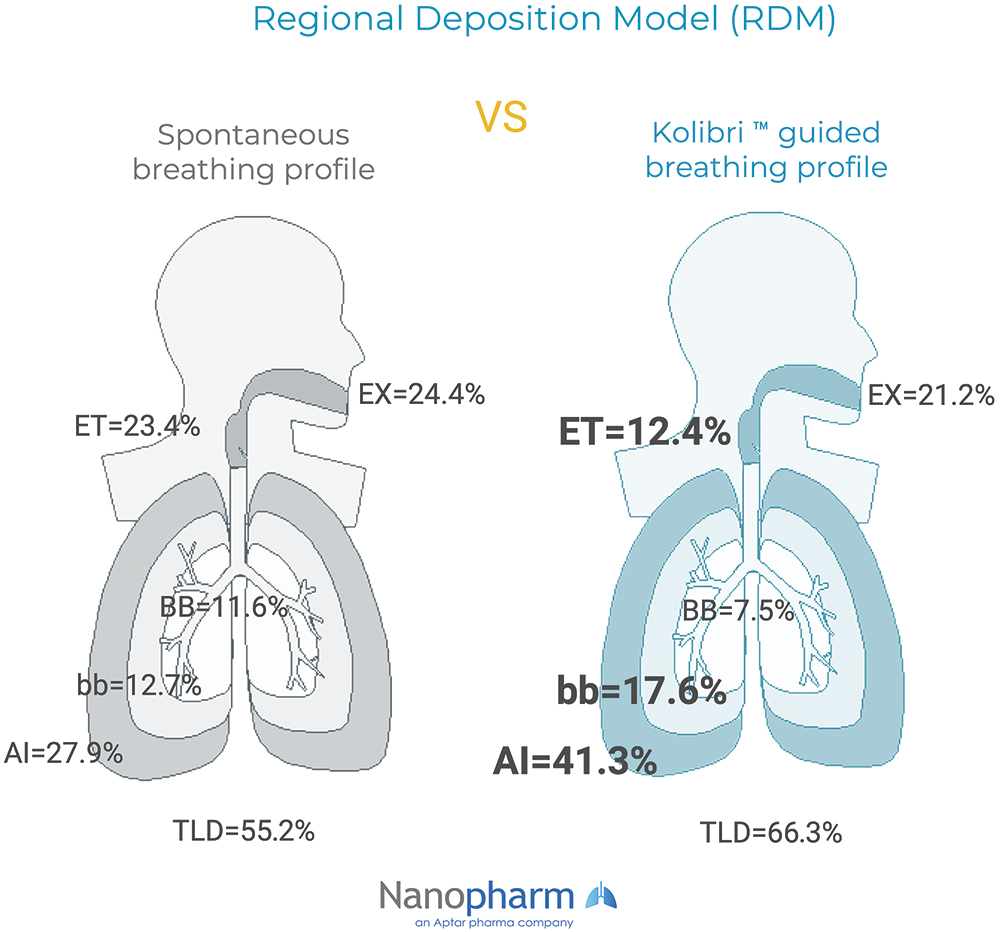
Figure 4: RDM predicted lung deposition (mean % of DD) in the extra thoracic (ET), tracheobronchial (BB; generations 1-8), bronchiolar (bb; generations 9-15), alveolar-interstitial (AI; generations 16-23) regions and the exhaled fraction (EX). Total Lung Deposition (TLD), equal to the sum of BB, bb and AI.
Functional Respiratory Imaging
The functional respiratory imaging conducted by Fluidda was based on 3D lung models taken from computed tomography (CT) scans. It simulated deposition by applying computational fluid dynamics to two disease models: cystic fibrosis (CF) and non-cystic fibrosis bronchiectasis (NCFB), as shown in Figure 5.
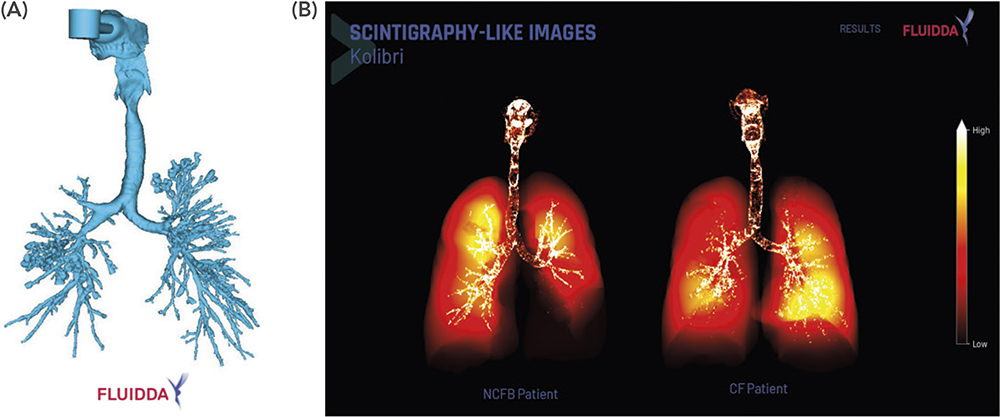
Figure 5: Reverse engineered CT scan of Kolibri™ virtually coupled to the mouth of a CF patient (A); FRI predicted lung deposition in scintigraphy-like images (B).
Compared with the spontaneous profile, the Kolibri guided breathing profile demonstrated:
- Lower extra-thoracic (16.5% and 19.0% versus 25.2% and 24.2%) and higher intra-thoracic (78.2% and 77.1% versus 58.0% and 62.4%) deposition in both CF and NCFB patients
- Higher peripheral deposition – 58.8% (CF) and 51.4% (NCFB).
In these two in silico models, including both healthy and diseased lung models, the realistic Kolibri guided breathing profile demonstrated a more favourable pattern for deep lung deposition compared with a spontaneous unguided breathing profile.
THE POWER OF COLLABORATION: UNLOCKING NEW POSSIBILITIES
The current synergy between PULMOTREE and Aptar enables a seamless integration of cutting-edge nebuliser technology with realistic breathing profiles, providing a comprehensive approach to aerosol characterisation. By combining in vitro and in silico studies, this partnership creates a robust ecosystem for pharmaceutical partners seeking to optimise the combination of their inhalation formulations and the chosen device. The collaboration offers multiple advantages for partners, including:
- Risk reduction in clinical development by providing access to realistic and representative data from in vitro and in silico testing
- Optimised drug-nebuliser matching and tailoring, ensuring safer and better therapeutic outcomes
- Regulatory advantages, including realistic data that can improve in vitro/in vivo correlation, supporting regulatory submissions.
CONCLUSION: A STEP FORWARD FOR NEBULISED BIOLOGICS
By leveraging Kolibri’s guided inhalation technology and its strong industry collaboration with Aptar, PULMOTREE is transforming the respiratory delivery of biologics. The fast and seamless integration of real patient data into drug development enables improved nebuliser performance, optimised aerosol deposition and, ultimately, enhanced clinical outcomes. As the industry moves toward more efficient and personalised drug-device combination products in respiratory drug delivery, PULMOTREE and Aptar stand at the forefront of this transformation.
REFERENCES
- Brand P et al, “Total deposition of therapeutic particles during spontaneous and controlled inhalations”. J Pharm Sci, 2000, Vol 89(6), pp 724–731.
- Dantas C, Foerner P, Krueger U, “Examining User Breathing Patterns During Nebulization with a Guided Inhalation Maneuver”. Proc RDD 2024, Vol 1, pp 384–387.
- Dantas C et al, “Assessing the impact of realistic breathing profiles in aerosol deposition using in-silico methods”. DDL Proc, 2024, Vol 35, pp 395–398.

