To Issue 172
Citation: Lock D, “Rethinking GLP-1 Delivery Devices: Designing for Users Beyond Diabetes”. ONdrugDelivery, Issue 172 (May 2025), pp 62–67.
Dan Lock explores how the experiences and motivations of individuals using glucagon-like peptide 1 drugs for weight loss may differ from those managing diabetes or heart disease. Focusing on behavioural insights and understanding the specific challenges faced by people using these therapies for weight management can lead to more tailored drug delivery solutions that could enhance user engagement and adherence, and even disrupt the market leaders.
Glucagon-like peptide 1 (GLP-1) drugs are revolutionising weight management – but with more than half of users discontinuing their treatment within months,1 are current drug delivery solutions failing them? By rethinking the drug delivery devices used with GLP-1s, is it possible to improve adherence and unlock the full potential of GLP-1 therapies?
The first injection pens, such as the NovoPen (Novo Nordisk) launched in 1985, were designed to deliver insulin to people with Type 1 diabetes (T1D). The design of these pens is the result of years of iteration, including human factors research focusing on the needs of users with the greatest accessibility challenges across a diverse user population that ranges from children – who may need help from caregivers – to elderly adults with conditions such as arthritis, neuropathy or visual impairments.
“AS GLP-1s – ORIGINALLY INTENDED TO TREAT TYPE 2 DIABETES (T2D) AND OFTEN DELIVERED WITH INJECTION PENS DEVELOPED FOR INSULIN – ARE INCREASINGLY USED TO TREAT OBESITY, PERHAPS IT IS WORTH RETHINKING THE BASIS OF THESE DESIGNS TO ACCOMMODATE A USER GROUP THAT MAY HAVE DIFFERENT REQUIREMENTS.”
People with diabetes depend on these devices for survival, and the devices have been optimised accordingly for their ease of use, safety and reliability. However, as GLP-1s – originally intended to treat Type 2 diabetes (T2D) and often delivered with injection pens developed for insulin – are increasingly used to treat obesity, perhaps it is worth rethinking the basis of these designs to accommodate a user group that may have different requirements. Could poor adherence to GLP-1 drugs among those using it for weight loss (30% stop within four weeks without achieving clinically meaningful weight loss2) be explained, in part, by a user experience that does not cater to the specific needs of this population?
MARKET TRENDS IN GLP-1 USAGE
Recent market data suggest that around 5% of US adults (approximately 12.5 million people) have used or are currently using GLP-1s for weight loss alone.3 This is higher than the 7.4 million4 people (both adults and children) using insulin and equal to the 5% who have used or are currently using GLP-1s solely to treat a chronic condition such as diabetes or heart disease – approximately 3% are using GLP-1s to both treat a chronic condition and to lose weight. The comparative uptake of GLP-1s and insulin among US adults is shown in Figure 1.
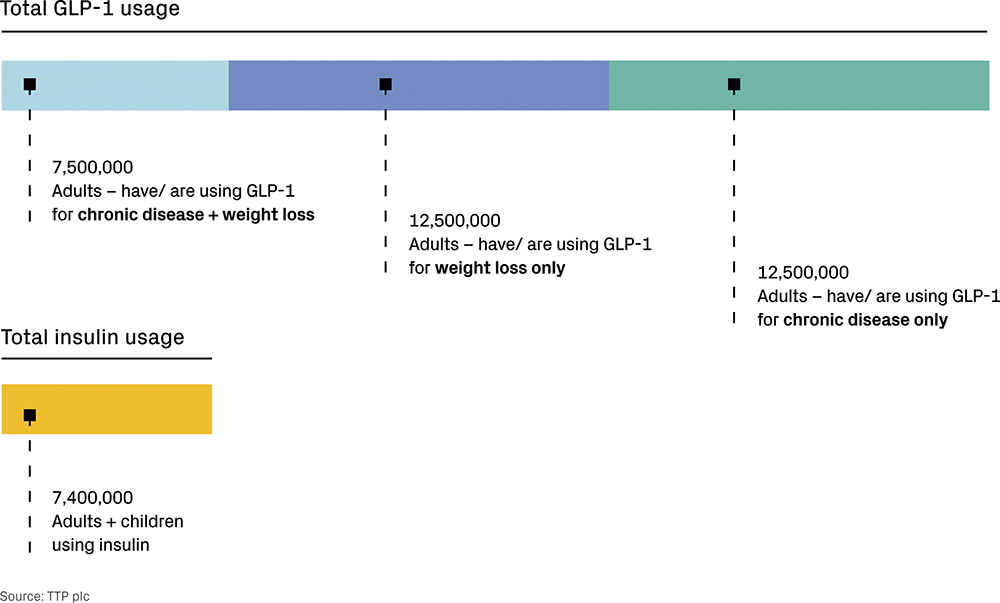
Figure 1: Usage of GLP-1s versus insulin.
As T2D is often associated with having a high body mass index, one would be forgiven for assuming that the needs of people who use GLP-1s for weight loss and those using it to treat diabetes would be very similar, but recent market research suggests a number of significant differences:5
- The majority of people using GLP-1s for weight loss are in their 30s to 50s (often as part of a wider strategy to be proactive about their health), whereas those using GLP-1s for diabetes control are more likely to be over 60.
- People using GLP-1s for weight loss are twice as likely to have a high income, whereas those using GLP-1s for diabetes control tend to be less well-off on average.
- A greater proportion of those using GLP-1s to target a weight loss of <7 kg are younger (teens to 30s) compared with those using GLP-1s to target a weight loss >7 kg.
- Of those targeting a weight loss of <7 kg, two-thirds already actively manage their health in some way, compared with only half of those targeting a higher amount.
These differences in user profiles are outlined in Table 1, and the trends are accelerating – the percentage of users prescribed GLP-1s exclusively for weight loss grew from 10% in October 2022 to 43% in October 2023. Yet, in many countries the devices being used to deliver GLP-1s are the same for both the weight loss and T2D populations and, in many cases, were originally designed for delivering insulin, such as Novo Nordisk’s FlexTouch pen (Wegovy, semaglutide) or Eli Lilly’s KwikPen (Mounjaro, tirzepatide) outside the US.
| User profile | GLP-1 for Weight Loss | GLP-1 for Diabetes Control |
| Average age | 30s to 50s | 60+ |
| Average income | Twice as likely to be high-income earners | Tend to be less well-off |
| Health management mindset | More proactive – GLP-1 is often part of a broader self-care strategy | More reactive – GLP-1 is used to manage a chronic condition |
Table 1: Differences in user profiles.
UNIQUE REQUIREMENTS
Designing effective GLP-1 delivery solutions requires a deep understanding of the people who use them. While regulatory standards ensure safety and functionality, addressing the unique needs of GLP-1 users for weight loss offers an opportunity to go beyond compliance, creating solutions that are not only practical but also deeply engaging.
This user group presents distinct challenges and opportunities for design. Unlike patients with diabetes, many of whom are well-acquainted with managing complex medication regimens, GLP-1 users for weight loss are often new to injecting and may have different levels of medical literacy, motivations and expectations. By tailoring the user experience to their specific profiles, it is possible to improve adherence, support better outcomes and position these devices as tools for health empowerment.
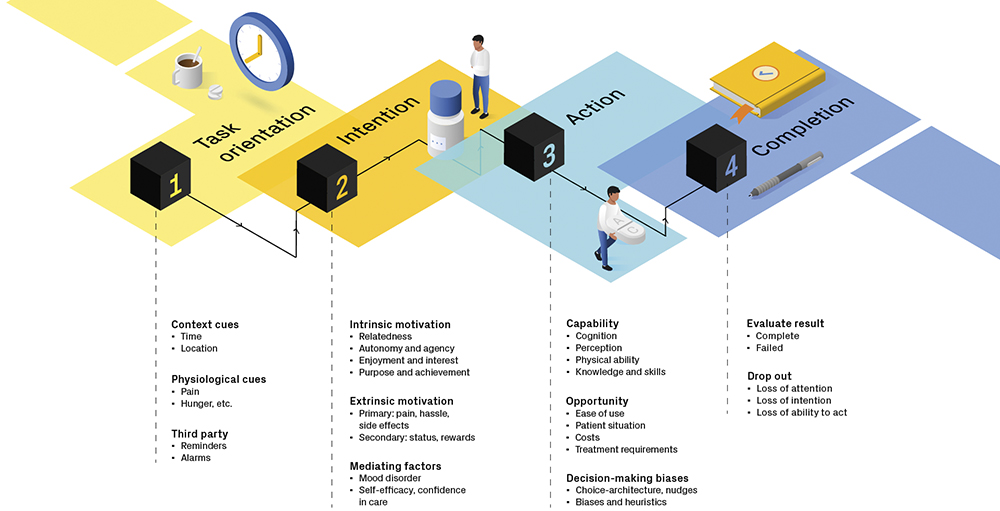
Figure 2: User engagement framework.
By developing a user-engagement framework6 that can be used to generate ideas for behavioural interventions at all stages of a task, it is possible to ensure that products resonate on a practical, emotional and aspirational level (Figure 2).This approach, grounded in behavioural science, addresses real-world barriers to medication adherence by mapping engagement touchpoints to drivers of motivation, providing a tool for design teams to think through problems and create solutions that not only function well but also encourage user participation. The challenges presented by using GLP-1s for weight loss – and the opportunities that solving those challenges offer – are summarised in Table 2.
| Challenges | Opportunities |
| Greater anxiety around injections | Lower need for portability |
| Poor adherence levels | Fewer comorbidities affecting capability |
| Users lack medical literacy | Users have a goal-oriented mindset |
Table 2: Challenges and opportunities for GLP-1 drug delivery devices.
CHALLENGES
Greater Anxiety Around Injections
People with diabetes often become familiar with managing complex medication regimens due to the ongoing need for blood glucose control. Frequent injections – sometimes four to five times a day – can lead to a level of routine and familiarity with the process, shaped by frequent exposure. For some, the level of comfort even extends to injecting through clothing or in public settings, practices that have been studied for safety. In fact, some diabetes associations no longer emphasise the use of alcohol wipes before injection as part of standard practice.7
“THE LESS FREQUENT NEED FOR INJECTIONS CAN MEAN THAT USERS DO NOT DEVELOP THE SAME LEVEL OF COMFORT OR ROUTINE, WHICH MAY INFLUENCE THEIR OVERALL EXPERIENCE AND ADHERENCE.”
However, for those on a weekly injection schedule, such familiarity may be harder to achieve. The less frequent need for injections can mean that users do not develop the same level of comfort or routine, which may influence their overall experience and adherence. GLP-1 users are often new to injecting and may feel anxious or reluctant about the process. As GLP-1s have moved towards weekly dosing, users are less likely to experience the desensitisation that comes from repeated exposure.
One way to tackle the issue is to reduce exposure by making injections even less frequent. Companies are investing in GLP-1 formulations and devices that allow for a lower injection frequency, such as quarterly injections. Developing devices that manage higher dose volumes, increased viscosity and larger needle diameters represent engineering challenges that are the subject of a great deal of ongoing research.
However, there are also ways to help anxious users feel in control while maintaining the current injection schedule. A review of posts by users on social media indicates that needle phobia is not necessarily as straightforward as not wanting to see the needle, as one Reddit user commented: “I am so scared to take my first shot because I can’t see the needle. Ozempic needles I can see, so I have control. I have a fear of needles so I’m having major anxiety.” For many, the uncertainty, tension and sudden release of an autoinjector is more anxiety inducing than injecting with an exposed needle. This need to be in control, or “autonomy”, is the most essential driver of intrinsic motivation.8
The challenge, therefore, is to design a drug delivery solution that maximises that sense of autonomy while also maintaining the simple workflow that autoinjectors offer. There are a number of concepts that could be explored, such as adding a retractable window that allows the user to see the needle only if they want to or reworking the trigger mechanism to ramp up smoothly rather than the current characteristic “snap”.
Poor Adherence Levels
For people with diabetes, insulin injections are critical. A study comparing adherence between people with T2D that use insulin and those using GLP-1s found that insulin users had an adherence level of around 90% compared with around 74% for those using GLP-1s (both oral and injectable versions).9
For those using GLP-1s for weight loss, injections are a choice. As a consequence, levels of adherence are lower than for people with diabetes, as demonstrated by another study that applied the same metric of adherence to people using GLP-1s to treat obesity: 40.1% for Ozempic (semaglutide, Novo Nordisk), 31.5% for weekly injectable Wegovy and just 15% for daily injectable Saxenda (liraglutide, Novo Nordisk).10 This suggests that greater expectation management is required to maintain users’ faith in the value and purpose of what they are doing. These differences in adherence rates across various medications and use cases are illustrated in Figure 3.
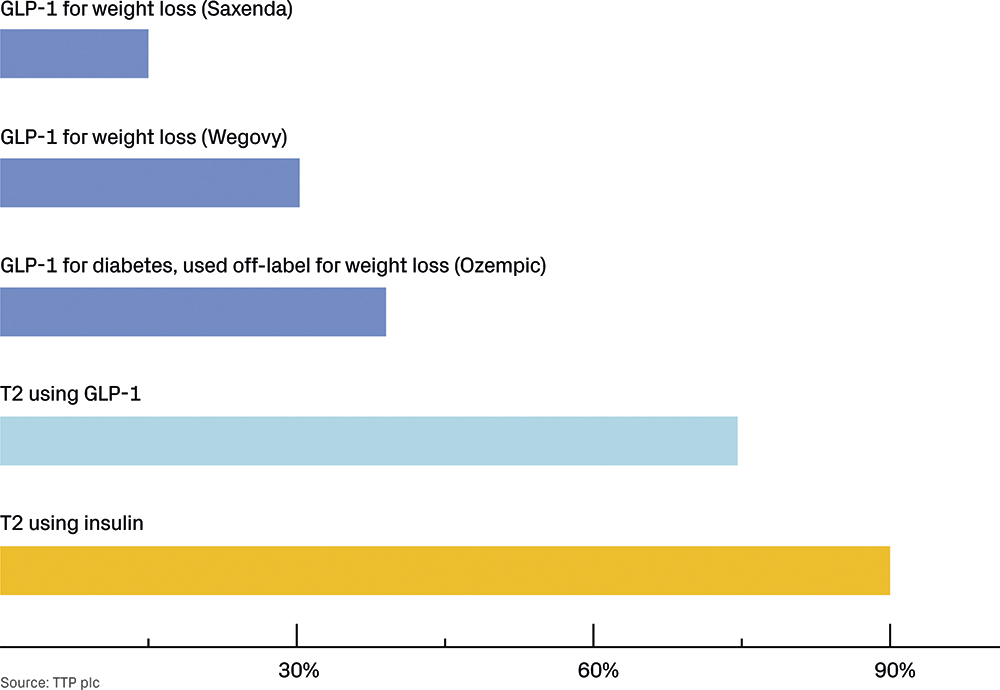
Figure 3: Adherence rate.
Adherence is generally affected by a combination of capability (strength, dexterity, cognitive powers), opportunity (complexity, cost, availability) and motivation (intrinsic drivers, rewards, general mood).11 Based on this, it is possible to calculate relative differences in likely adherence with a quantitative analysis of typical disease impact, patient demographics, drug effects and device operating principles.12 By systematically analysing a drug delivery solution, it is possible to identify opportunities for improvement and determine the cost-benefit of investing in a wide range of design features.
Lacking Medical Literacy
People with diabetes have a steep learning curve upon diagnosis but, over time, many develop a deep understanding of their condition and the way their bodies respond to carbohydrates, insulin, exercise and other factors. Training courses for patients diagnosed with T1D are practically oriented and emphasise the critical importance of proper nutrition and recognising and managing the signs of both low and high blood sugar.
GLP-1 users may not have such earnest study and medical knowledge thrust upon them. They are less likely to rely on authoritative sources of medical information and more likely to make risky medical choices based on opinions and experiences shared online. For example, “stacking” different brands of GLP-1 medication, using more than the prescribed dose or changing dosing intervals without reference to a medical professional – as one Redditor commented: “I did 10 my first shot, then 12. I just now gave myself 15 for week 3. If all goes well, I’ll up it to 20 next week. I’m impatient and this is too expensive to be overly cautious.”
A balance must be struck between creating a comfortable experience – such as minimising medical aesthetics and any stigmatising elements – and ensuring that users fully understand their treatment and approach it with the seriousness it requires. A companion app that helps to guide new users through the first few weeks of learning the device and titrating the dose and that explains what to expect, what to look out for and what kind of weight loss is reasonable, may be one way to help to manage expectations and keep users safe. Another might be electronically preventing non-standard dosing schedules or quantities.
The problem is also partly driven by cost. Many users find branded GLP-1s are not affordable and turn to lower cost sources, such as getting semaglutide made up by a compounding pharmacy (often requesting additions to the formula such as vitamin B12) – a practice that is not recommended by the US FDA.13 This may decrease as the first generic versions of GLP-1 drugs become available, which started to get FDA approval in late 2024. While there are typically serious limitations to what changes can be made to the method of operation for drug delivery devices, there may also be an opportunity for competing products to differentiate themselves through access to high-quality patient support materials and moderated forums.
OPPORTUNITIES
Less Need for Portability
Insulin pens for rapid-acting insulin must be portable so that users can easily carry them throughout the day. In contrast, pens for long-acting insulin, used once or twice daily, have fewer portability requirements, although similar devices are often used for both.
GLP-1 treatments for weight loss are typically injected at home once daily or weekly, meaning that portability is even less critical unless users are travelling. Reduced portability requirements allow for designs that are more engaging, supportive and ergonomic, with more premium aesthetics driven by a consumer health mindset.
“BY CONDUCTING USER PREFERENCE STUDIES BEFORE DETAILED ENGINEERING BEGINS, DESIGNERS CAN LEARN THE ACCEPTABLE LIMITS FOR DEVICE SIZE AND CAN MAKE INFORMED TRADE-OFFS IF INTERNAL COMPONENTS REQUIRE ADJUSTMENTS.”
By conducting user preference studies before detailed engineering begins, designers can learn the acceptable limits for device size and can make informed trade-offs if internal components require adjustments. This approach ensures that the final product closely matches user expectations and, in subsequent user evaluations, is more likely to be significantly preferred over alternatives.
However, sometimes improvements to user experience come from combining devices with digital technologies, such as smartphone apps, without changing the injection device itself. Providing better support, especially in the initial phase of treatments such as GLP-1s, can greatly improve the user experience, reduce anxiety and help users adhere to their treatment plans.
Fewer Comorbidities Affecting Capability
People with diabetes are more likely to suffer from peripheral neuropathy (29.1% of T1D, 42.2% of T2D) and retinopathy (28.4% of T1D, 23.8% of T2D),14 among other things. Devices must include high-contrast labels and tactile features for those with visual or dexterity impairments. Those using GLP-1s for weight loss may deal with obesity or hypertension but are less likely to have severe visual or dexterity issues. This means that designers may have a little more leeway and flexibility on aesthetic aspects of design.
Donald Norman coined the concept of “Emotional Design”,15 which proposes that users engage with products at three levels:
- Visceral: The instinctive level comprising gut reactions driven by evolutionarily derived aesthetic preferences.
- Behavioural: The purely utilitarian level that is focused on functionality and ease of use.
- Reflective: The intellectual level that incorporates cultural status, meaning, sentimental value and so on.
Medical device development focuses, correctly, on what Norman called the “Behavioural” level. However, there is also strong evidence that products with aesthetic appeal lead to enhanced usability,16 perhaps because users are more inclined to invest their time in learning how to use it. Often, when designers cannot understand the popularity of a product, it is because they have neglected to consider one of these levels.
Having the freedom to invest in designs that tap into each level, particularly the “Reflective” level, could help with engagement. One could envisage ways in which this freedom could be used to create something more personal, or even a range of options to cover the design needs of different user segments and cultures.
Given the success of consumer-oriented wellness products and services, adopting a less medical “feel” for the experience and tuning in more to the aesthetics and sensibilities common to lifestyle products could improve user adoption. Taking inspiration from fitness and wellness products, such as Strava, Whoop and Noom, devices could bolster users’ self-identity as someone proactively managing their health, fostering a sense of empowerment and engagement.
Goal-Oriented Mindset
People with T2D are typically prescribed GLP-1s to stimulate their pancreas and to ward off worsening symptoms that may lead them to require using insulin down the line. They anticipate chronic use of the drug – at least until more intensive treatment becomes necessary.
In contrast, people using GLP-1s for weight loss may be more focused on achieving a specific goal: they hope to resolve an issue – obesity – rather than simply maintaining or prolonging their current health status. Their motivations can vary, driven by concerns such as health (50%), appearance (35%) or mood (15%).17 As a result, they may not perceive their need as strictly medical and so may be put off by products with overtly medical aesthetics. Additionally, weight loss is generally seen as having a definitive endpoint, leading users to (rightly or wrongly) expect that their treatment can be tapered down or discontinued once their goal is achieved. This goal-oriented approach brings a different psychological perspective compared with managing other chronic conditions.
“A WEIGHT-LOSS FOCUSED GLP-1 DEVICE SHOULD EMPHASISE PROGRESSION AND MOVEMENT TOWARDS A GOAL, WHICH MAY DIFFER FROM DESIGNS FOR T2D USERS.”
A weight-loss focused GLP-1 device should emphasise progression and movement towards a goal, which may differ from designs for T2D users. Targeting specific segments – such as health, appearance and mood – could further refine device positioning. For example, athlete-focused glucose monitors have been successfully adapted to prioritise performance optimisation over strictly medical functionality, offering a tailored approach that is distinct from those designed for diabetes management, although careful analysis and testing are needed so as not to undermine safety critical elements of the design or workflow.
CONCLUSION
By addressing the unique requirements of people using GLP-1s for weight loss, device teams can develop solutions that support better adherence and create a positive, engaging user experience. It is clear that the early integration of engagement strategies can lead to devices that resonate with users as tools for health empowerment.
While this article has focused on the differing requirements for people using GLP-1s for weight loss, the list of proposed indications for GLP-1s grows seemingly on a daily basis, with research currently ongoing in neurodegenerative disease, chronic kidney disease and liver disease. Each of these patient groups will have unique needs, underscoring the importance of designing a diverse range of devices tailored to the specific requirements of each segment.
REFERENCES
- Gasoyan H et al, “Early- and later-stage persistence with antiobesity medications: A retrospective cohort study”. Obesity, 2024, Vol 32(3), pp 486–493.
- “Real-World Trends in GLP-1 Treatment Persistence and Prescribing for Weight Management”. Blue Health Intelligence, May 2024.
- Montero A et al, “KFF Health Tracking Poll May 2024: The Public’s Use and Views of GLP-1 Drugs”. KFF, May 2024.
- Grassley CE, Wyden R, “Insulin: Examining the Factors Driving the Rising Cost of a Century OldDrug”. Report, US Senate Finance Committee, 2021.
- “Consumers Using Glp-1 Medications for Weight Loss Skew Younger and Higher Income, Numerator Reports”. Press Release, Numerator, Nov 2023.
- Lock D, “Designing for user engagement in medical technologies – a framework people want to use”. TTP, Feb 2025.
- Fleming DR et al, “The Safety of Injecting Insulin Through Clothing”. Diabetes Care, 1997, Vol 20(3), pp 244–247.
- Deci EL, Ryan RM, “The ‘What’ and ‘Why’ of Goal Pursuits: Human Needs and the Self- Determination of Behavior”. Psychol Inq, 2000, Vol 11(4), pp 227–268.
- Palanca A et al, “Real-World Evaluation of GLP-1 Receptor Agonist Therapy Persistence, Adherence and Therapeutic Inertia Among Obese Adults with Type 2 Diabetes”. Diabetes Ther, 2023, Vol 14, pp 723–736.
- Gleason PP et al, “Real-world persistence and adherence to glucagon-like peptide-1 receptor agonists among obese commercially insured adults without diabetes”. JMCP, 2024, Vol 30(8), pp 860–867.
- Jackson C et al, “Applying COM-B to medication adherence: A suggested framework for research and interventions”. EHP, 2014, Vol 16(1), pp 7–17.
- Lock D, “Design for adherence: a model for predicting and improving medication adherence”. TTP, Sep 2023.
- “FDA’s Concerns with Unapproved GLP-1 Drugs Used for Weight Loss”. US FDA, Dec 2024.
- Pfannkuche A et al, “Prevalence and risk factors of diabetic peripheral neuropathy in a diabetics cohort: Register initiative ‘diabetes and nerves’”. Endocr Metab Sci, 2020, Vol 1(1–2), art 100053.
- Norman D, “Emotional Design: Why We Love (or Hate) Everyday Things”. Basic Books, Mar 2007.
- Kurosu M, Kashimura K, “Apparent usability vs. inherent usability: experimental analysis on the determinants of the apparent usability”. CHI ‘95: Conference Companion on Human Factors in Computing Systems, 1995, pp 292–293.
- O’Brien K et al, “Reasons for wanting to lose weight: different strokes for different folks”. Eat Behav, 2007, Vol 8(1), pp 132–135.

