To Issue 159
Citation: Parker M, Lyons S, Lyness A, “Scaling GLP-1 Drugs Without a Mountain of Waste”. ONdrugDelivery, Issue 159 (Apr/May 2024), pp 12–15.
Matt Parker, Simon Lyons and Alex Lyness, look at how the continued success of glucagon-like peptide-1 drugs is bringing into focus the need for environmentally sustainable self-injection systems. They discuss whether reloadable autoinjectors could be the way to boost sustainability, lower cost per dose, and match the scale and rate of adoption of these new therapies.
This year, glucagon-like peptide-1 (GLP-1) analogues are set to overtake programmed cell death protein 1 (PD-1) inhibitors as the best-selling class of drugs. By 2030, hundreds of millions of patients worldwide may rely on GLP-1 drugs to manage their health, some for a period of years, others perhaps indefinitely.
“The requirements of GLP-1 drugs are also different from those for which existing reusable autoinjectors were developed.”
The initially unforeseen clinical success of GLP-1 drugs and their rate of adoption has already led to a number of challenges for the industry, from the ability to manufacture the drug to bottlenecks due to fill-finish capacity to payers tackling reimbursement. Assuming these are resolved in the coming years, there are still significant questions about how we can address the environmental sustainability challenges created by this new market and the vast patient population.
In the short term, scaling the production of proven, existing single-use autoinjector and insulin-style or cartridge-based injector pen technologies is likely the best option to meet the rate of demand. Cartridge-based injector pens offer one route to reduce the environmental impact as, compared with single-use devices, they typically deliver up to four doses with only the needle hub discarded after each dose. But these advantages are offset by the usability challenges compared with single-use autoinjectors, including dose setting, an exposed needle and an increased number of steps to dispose of and replace it.
Longer term, can traditional pens designed to deliver insulin work for the needs of a patient on GLP-1? Is it acceptable to follow the same single-use disposable path for such a large market that is set to grow each year to 2030? Will this approach be acceptable to patients when more convenient and sustainable solutions could be available?1
For some years now, the industry has been weighing up the potential of a reusable, durable autoinjector. This would take the form of a handheld device that reuses the power system each time but delivers the drug from a single-use disposable as a route to lower the environmental impact per dose. This disposable would contain a prefilled syringe (PFS) that is in drug and patient contact. While there has been some progress in this realm, the requirements of GLP-1 drugs are also different from those for which existing reusable autoinjectors were developed.
This article explores this premise further and proposes that simple mechanically driven reloadable autoinjectors may balance environmental sustainability and economic opportunities to create the best of both worlds.
THE GROWTH CURVE AHEAD
The metabolic diseases of diabetes and now, increasingly, weight management – collectively known as diabesity – are currently driving unprecedented demand for GLP-1 drugs. According to one estimate, the number of patients to be treated in the US is on track to reach 30 million by 2030.2 While some of the demand will be met with orally delivered treatments,3 the increase in the US alone could equate to one billion injections per year (Figure 1).
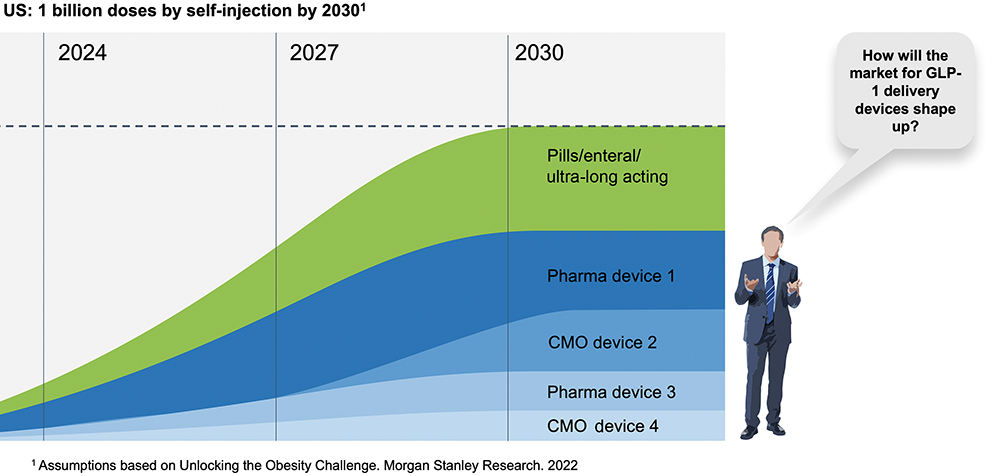
Figure 1: Growth in GLP-1 drugs and devices to equate to around 1 billion single-use autoinjectors per year.
Although welcomed by patients, the rapid growth of GLP-1 drugs over the next decade is bringing into focus the need for environmentally sustainable solutions for self-injection. An additional one billion typical single-use autoinjectors discarded after use could equate to 35,000 tonnes or 50,000 cubic metres of waste – and enough devices to circle the globe 3.5 times (Figure 2).
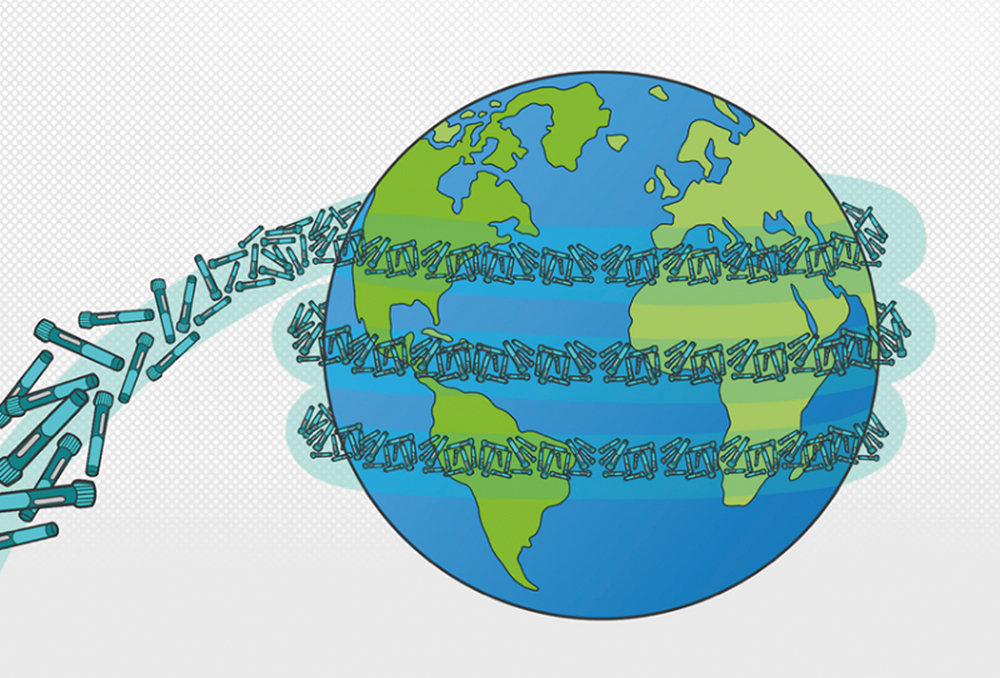
Figure 2: The success of GLP-1 drugs may have been unforeseen but the waste they are set to generate – through using existing single-use injection systems – certainly is not. Industry players must work together to innovate new delivery solutions to meet this challenge.
“An additional one billion typical single-use autoinjectors discarded after use could equate to 35,000 tonnes or 50,000 cubic metres of waste – and enough devices to circle the globe 3.5 times.”
REDUCING ENVIRONMENTAL IMPACT
These staggering numbers clearly show that environmental sustainability should be considered from the outset of future drug product launches in this category. To justify the investment into a new drug delivery platform often requires an order of magnitude improvement over incumbent devices, and there are only limited ways this can be achieved with the design of current products and processes.
An obvious method to enhance sustainability would be through minimising the amount of material used during manufacture. While some opportunities may exist here, many legacy products have been optimised for part count, material usage, manufacturing method and assembly, so the benefits from this approach alone are limited. An alternative would be to develop an autoinjector made with more sustainable materials from the outset, such as the Eco-inject® platform. The number of bio-based and biodegradable polymers is increasing steadily, making it possible to meet mechanical performance requirements. However, these new materials still command a premium over conventional medical grades, which will impact cost per dose until they are available and adopted at scale.
Full circularity is a more radical approach focused on maximising the takeback and recycling of a device through material choices and design for disassembly at end of life. The infrastructure, logistics and regulation are taking shape, albeit at different rates in different territories, and takeback schemes are being trialled in various industries. Health Beacon (Dublin, Ireland) is one of the leaders in this field and tackling the complexities required for collecting devices with sharps that have been in contact with both the drug and the patient. Circularity via recycling or takeback is being piloted but, as well as being physically possible, it must also be economically viable at scale if the benefits are to be relished.
A RELOADABLE AND REUSABLE AUTOINJECTOR?
One potential option to tackle this challenge is to develop an autoinjector intended to be reloaded and reused. Reusing the drive system and several components across multiple deliveries is a practical way to significantly lower the environmental impact per dose. The user is still required to load the device between uses but – unlike traditional cartridge-based pens – the needle would not be seen by a patient prior to an injection and shielded post-injection, features that reduce anxiety and increase safety during self-injection.
“A mechanical reloadable design brings clear environmental sustainability benefits but, conversely creates unique design challenges.”
To date, existing reusable platforms in this area have typically been designed around powerful electromechanical drive systems to drive (often viscous) biological drugs. This results in costly battery-powered devices that have then sought to include additional functionality and connected features to justify the increased costs. In the context of GLP-1, rather than repurposing this approach, the needs of the new patient population and scale of the market should be taken into account when developing the correct delivery solution.
GLP-1s tend to have favourable properties for injection, such as low viscosity and relatively small dose volumes that can be delivered from a PFS via a spring-powered device. For a drug regime like that of many GLP-1s, where patients administer a dose weekly, it is easy to see how simple reloadable autoinjectors could offer a significant improvement in environmental sustainability performance over single-use devices.
To maximise the environmental benefit of this approach, many of the complex high-value components (actuator, needle safety, ergonomic handle, etc.) would be integrated into the reusable section of the device. The disposable section would appear as a simple cassette that would house the PFS and enable the user to easily reload the autoinjector.
UNIQUE DESIGN CHALLENGES TO OVERCOME
A mechanical reloadable design brings clear environmental sustainability benefits but, conversely, creates unique design challenges. Some are technical, such as part durability, human error during user loading versus preloaded, ensuring a lockout at end of life of the device, etc. Others are more nuanced and require significant design innovation to develop a device that meets the requirements for sustainability while, at the same time, satisfying the user and being compatible with existing manufacturing methods and fill-finish equipment.
Primary Containment
When it comes to the primary containment, dedicated innovation is best avoided. Seeking a custom packaging solution introduces technical, logistical and commercial challenges to both the manufacturing and fill-finish operations. Instead, all efforts should be put into integrating an off-the-shelf PFS solution that will have been optimised, tested and already proven scalable into the reloadable autoinjector design.
“Simple reloadable and reusable autoinjectors are a pragmatic solution that balances environmental sustainability and economics in a way that favours adoption and scalability.”
User Acceptance and Adherence
To gain adoption in a mass market dominated by single-use devices, a reloadable autoinjector should offer a familiar format and design affordances, identical or similar user steps for injection and intuitive reloading steps. In practice, it should offer familiar haptic cues so that users can recall functional steps. As far as possible, these steps should follow current accepted practice; any new steps should draw on recognisable and familiar affordances associated with reloading or refreshing a device. Examples can be drawn from our everyday lives and the many different coffee machines and coffee pods we have become accustomed to using to prepare our daily dose of caffeine with minimal training.
Device Simplicity
It may at first appear that a reloadable autoinjector will increase complexity for the user. Typically, additional steps are required to swap over a PFS in a reusable device. To reduce the number of steps, the functions required could be designed into a novel cassette design. Going a step further, an ideal solution would make reloading the autoinjector feel simpler and more worthwhile to the user than unpacking and throwing away a whole device after each use.
Regulatory
A device design with similar user steps and interaction cues to existing marketed devices would be preferred. There is precedent for US FDA-approved reusable autoinjectors intended for different therapeutic indications, for example: the SKYTROFA® autoinjector (Ascendis Pharma) containing the human growth hormone product lonapegsomatropin-tcgd; the Rebiject II® autoinjector (Merck KGaA), for use with the company’s Rebif® (interferon beta-1a) prefilled syringe in multiple sclerosis; and the Enbrel AutoTouch® autoinjector (Amgen), for use with the Enbrel Mini® (etanercept) single-dose prefilled cartridges in various forms of arthritis, ankylosing spondylitis and plaque psoriasis. Care should be taken to not deviate significantly from familiar device interactions or introduce handling steps not supported by extensive usability studies.
COMMERCIAL BENEFITS: COST PER DOSE AND SCALABILITY
Simple reloadable and reusable autoinjectors are also a pragmatic solution that balances environmental sustainability and economics in a way that favours adoption and scalability. Fundamentally, a smaller number of reloadable devices of comparable complexity to a single-use device will need to be manufactured and assembled to supply the same number of doses – or to keep up with the growing demand of the GLP-1 market. In a field saturated by single-use devices, reloadable autoinjectors also give pharmaceutical companies an opportunity to offer product differentiation while aligning with the growing demand for sustainability from users and legislators.
CONCLUSION
In the short to medium term, simple reloadable autoinjectors are a credible option to align environmental sustainability with commercial drivers, in particular the need to scale. To realise this opportunity, the stakeholders need to collaborate to resolve several interrelated design and manufacturing challenges. Many of these start with the needs of the end user, including anticipating the minimum device features expected, ensuring the injection experience does not deviate significantly and integrating the existing PFS solutions into the cassette design so it is intuitive and reliable to reload. The size of the GLP-1 market means that there are vast revenues to be generated for a number of pharma companies, albeit with a high degree of competition and difficulty around differentiation. It would be hoped that the societal benefit of these new drugs is to be combined with similar innovations in delivery that lead to scalable, reusable autoinjector solutions that ensure that the millions of patients, and the environments they live in, have a healthy and more sustainable outlook to 2030 and beyond.
REFERENCES
- Zhou A, Trujillo J, “Comparison of Usability, Accuracy, Preference, and Satisfaction Among Three Once-Weekly GLP-1 Receptor Agonist Pen Devices”. Diabetes Spectr, 2018, Vol 31(4), pp 359–366.
- “The increase in appetite for obesity drugs”. Research Analysis, J.P. Morgan, Nov 29, 2023.
- Boye K et al, “Patients’ preferences for once‐daily oral versus once‐weekly injectable diabetes medications: The REVISE study”. Diabetes Obes Metab, 2021, Vol 23(2), pp 508–519.

