Citation: Hlobik T, “Simplifying the Process of Developing Prefilled Syringes”. ONdrugDelivery Magazine, Issue 101 (Oct 2019), pp 76-79.
Tibor Hlobik describes how the use of cyclic olefin polymer delivery systems is gaining in popularity because of the benefits they have over glass systems for complex drug applications, and explains what this advance in technology can offer drug manufacturers.
Selecting the best materials for packaging and delivering drugs is critical in improving stability and performance risks. Sensitive drugs, and in particular biologics, are often complex entities and more challenging to administer. Choosing materials that present the lowest possible risk of interaction, such as low surface energy cyclic olefin barrels and barrier-coated elastomeric plungers, is critical for success.
Delivery systems based on cyclic olefin polymers (COPs) are therefore becoming increasingly popular because these materials can meet the challenges of providing the quality, safety and reliability needed for complex therapeutic applications.
Whether preparing for clinical trials or drug product commercialisation, there is now a wide platform of polymer systems commercially available and ready to use off-the-shelf, to meet unique user requirements, and complimentary support and service offerings to help navigate work required during development of products, for commercialisation and drug lifecycle management. The variety of options and treatment applications (Figure 1) include:
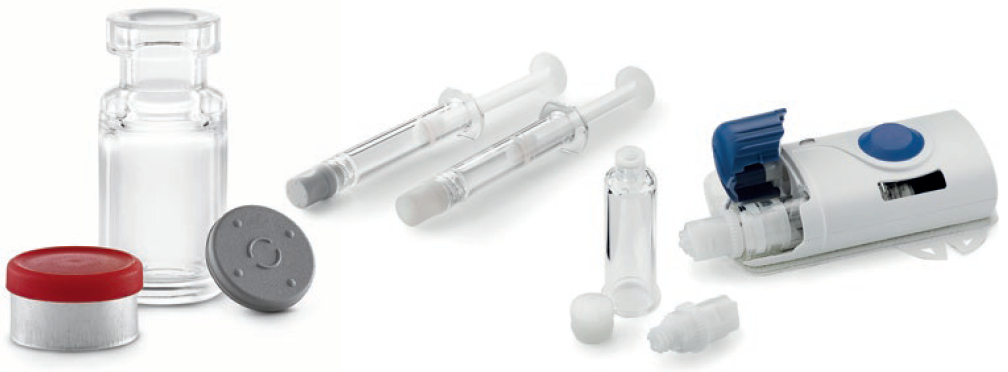
Figure 1: West’s offering of polymer base primary containers and delivery systems includes vials, prefillable syringes, and cartridges.
- Vial systems in a range of sizes with low extractables and low subvisible particles for storage at low temperatures for cell and gene therapies
- Small volume 0.5 mL and 1 mL prefillable Luer lock syringes used in ocular injections with ophthalmic treatments where there is high sensitivity to silicone oil
- 1 mL long and 2.25 mL prefillable insert needle syringes used to administer treatments with auto-injectors where syringe breakage is a concern for high viscosity drugs
- 3.5 mL and 10 mL cartridges for wearable large volume, on-body patient-controlled injectors that enable precise drug delivery over longer time periods for biologic pipeline drugs and intravenous to subcutaneous product conversions.
BENEFITS OF POLYMER SYRINGES
COPs offer advantages over glass thanks to a number of attributes and design features. High-quality COPs such as the Daikyo Crystal Zenith® syringe systems, are designed to overcome complex challenges, provide solutions for unique user requirements and add value to complex and sensitive biologics. This includes the absence of silicone oil in Daikyo Crystal Zenith® syringes, which results in decreased interaction with the drug product and enhanced cleanliness.
The Daikyo Crystal Zenith® Insert Needle Syringe system supplied with Flurotec® pistons is designed to maintain the purity, integrity and efficacy of premium biopharmaceutical therapies and can be coupled with an autoinjector device that provides greater patient convenience and ease of use through self-administration.
“West is uniquely positioned to provide fill/finish support services and can assist drug developers with small-scale sample preparation for product testing, line implementation at a customer site, third-party clinical and commercial filling, support testing and programme management.”
The syringe system minimises the potential contamination issues associated with other container materials and helps to reduce the risk of product interactions and performance issues. This helps to meet drug product performance needs that include:
- Tighter dimensional tolerances
- Higher break resistance
- Silicone oil-free barrels
- Tungsten and glue free
- Repeatable functional performance
- Higher fill volume threshold.
Ophthalmic applications present a whole new set of challenges. The majority of intravitreal injections are anti-VEGF drugs packaged in either a vial format, using either a polypropylene, heavily siliconised syringe, or a siliconised Luer lock prefilled syringe with attached needle. However, silicone oil has been associated with floaters in patients’ vision. The Daikyo Crystal Zenith® Luer Lock 0.5 mL syringe system can provide improved dose accuracy and higher final drug product quality. It is also silicone oil free, has low endotoxin limits, low sub-visible and visible particle limits, and offers repeatable functional performance.
MANAGING THE DEVELOPMENT OF COMPLEX PRODUCTS
West provides a complementary support service to help with the development of products for commercialisation and drug lifecycle management (Figure 2). Partnering with a company that not only provides the product, but also expertise in material science, primary packaging, delivery systems and experience in services to support pharmaceutical companies has many advantages.
Figure 2: With programmes designed for any stage of the drug development lifecycle and across all injectable formats, West can help you Simplify the Journey™.
A single partner can reduce development and supply risk, reduce total cost of ownership and accelerate your path to market. West now offers a comprehensive approach of integrated solutions to Simplify the Journey™ (Figure 3).
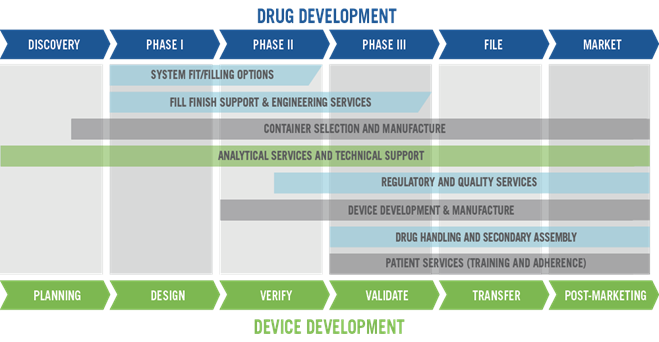
Figure 3: West can provide its clients with services throughout the drug development process.
Evaluation from Development to Commercialisation
It is important to document the early baseline verification data, typically generated in Phase II studies. These studies should show that the selected syringe system meets intended user requirements and should include performance evaluation, functionality characterisation as well as identify any associated chemical compatibility concerns.
The packaging and delivery system also needs to be evaluated against the baseline data and defined critical quality attributes (CQAs), before the drug product/device manufacturing is scaled-up for regulatory submission.
Phase III studies include performance testing of the drug product and syringe system during both real-time and accelerated conditions over the shelf-life of the product (Figure 4). Real-time conditions should include test intervals up to 36 months and accelerated conditions up to six months for functionality and performance, extractables and leachables, particulate and silicone oil analysis, dose accuracy and system integrity evaluations.
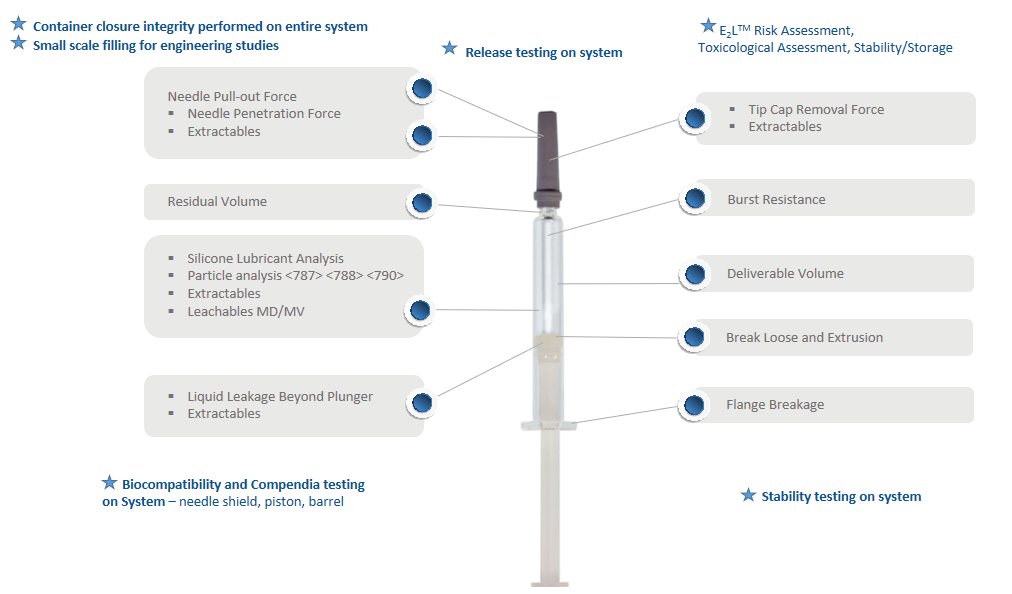
Figure 4: The Daikyo Crystal Zenith® Insert Needle Syringe system minimises the risk of contamination, product interactions and performance issues.
Other tests available, depending on programme requirements, include bacterial endotoxin, biocompatibility, and shipping simulations based on ASTM standards. The latter includes:
- Environmental conditioning
- Shipping and handling
- Low pressure, high altitude testing
- Post shipping analyses, including:
– Particles in solution
– Container closure integrity testing
– Helium leak detection
– Inspection (plunger placement and leakage, and needle shield or tip-cap placement and leakage).
“Partnering with a company that not only provides the product, but also expertise in material science, primary packaging, delivery systems and experience in services to support pharmaceutical companies has many advantages.”
Fill/Finishing Solutions
Finding fill/finish options for the broad variety of drug product packaging and containment options on the market can be a challenge for drug developers, particularly for novel formats that provide differentiation and improved patient experience but may have unique fill/finish requirements.
West is uniquely positioned to provide fill/finish support services and can assist drug developers with small-scale sample preparation for product testing, line implementation at a customer site, third-party clinical and commercial filling, support testing and programme management. This is because West has lab-scale fill/finish capability, an established third-party network and expertise in fill/finish requirements for innovative containment systems such as the Daikyo Crystal Zenith® polymer syringe technology.
Post-Market – Continuing the Journey
It is important to use the data and knowledge gained in product development and scale-up to keep improving the product and process. The standards that need to be met in technical transfers and change control processes are provided by the CQAs of the drug and the delivery system output requirements. In addition, ICH Q12 outlines the regulatory expectation for continuous management of a product over its lifecycle. The following services can also be offered:
- Device manufacturing and assembly
- Drug and device packaging solutions
- Device serialisation
- Drug handling, including cold storage
- Quality control release testing
- Regulatory services
- Support of tech transfers or change management
- Release testing based on customer requirement.
CONCLUSION
Greater scrutiny needs to be paid to the interaction between a drug and its container closure system. Drug stability over its shelf life, particulate burden, the prevention of breakage and ease of delivery are important factors to consider. In addition, regulatory agencies and pharmaceutical companies have increased quality expectations to enhance patient safety.
Through break resistance, superior functional performance, highly reduced extractables and the availability of sterile formats, polymer syringes present attractive benefits that are gaining increased attention from manufacturers seeking new answers to increasing drug delivery and administration challenges.
The West Integrated Solutions programme brings together West’s primary packaging, device, analytical, regulatory and contract manufacturing expertise in a single-source solution. With programmes designed for any stage of the drug development lifecycle and across all injectable formats, West can help you Simplify the Journey™ at any stage of drug development.
SmartDose® is a registered trademark of West Pharma. Services IL, Ltd., a subsidiary of West Pharmaceutical Services, Inc., in the United States and other jurisdictions. Crystal Zenith® is a registered trademark of Daikyo Seiko, Ltd. Daikyo Crystal Zenith® technology is licensed from Daikyo Seiko, Ltd.

