Citation: Schmidt Moeller C, “Subcuject WBI: Low-Cost, Larger Volume, High-Viscosity Wearable Bolus Injector – Using Standard Glass Primary Packaging”, ONdrugDelivery, Issue 120 (May 2021), pp 58–60.
Claus Schmidt Moeller introduces the company’s wearable bolus injector for single use and discusses the advantages of the device.
HIGHER VOLUME DELIVERY OPTIONS
Subcutaneous injection of volumes above 2 mL is becoming a reality. To administer this, a pharmaceutical company can choose one or more prefilled syringes, more than one autoinjector or a wearable injector.

Figure 1: The Subcuject WBI (5 mL).
The use of more than one device for a single injection sequence increases the risk of incorrect use by the user. Hence, a single injection device in the form of a wearable bolus injector may be preferable. However, several of the wearable injectors in development are either expensive to manufacture, use primary packaging materials that are not covered in standard stability documentation or involve additional user steps – such as assembly of parts. Furthermore, a number of wearable injectors in development are quite big in size due to the need to accommodate motors, gears and batteries or a large spring as the drive mechanism.
Subcuject has developed a purely mechanical wearable injector for single use (Figure 1), using forward osmosis as the drive mechanism and using a standard glass primary container. This achieves significant economic advantage, with the device cost expected to be in the range of a single-use autoinjector. Subcuject is currently collaborating with Phillips-Medisize to bring the wearable bolus injector (WBI) to market.
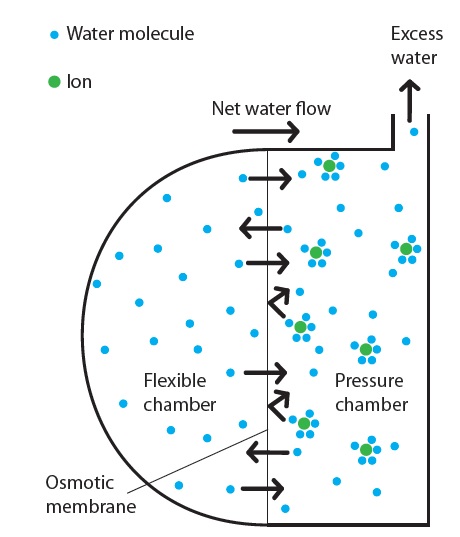
Figure 2: Schematic representation of the osmotic process in the Subcuject wearable injector.
SUBCUJECT WBI WORKING PRINCIPLE
“As pressure needs to be built up before the plunger starts moving, there is a soft injection start for increased patient comfort and minimised risk of impact damage in the primary container.”
The driving mechanism main elements are freshwater, salt and two semi-permeable forward osmosis membranes. Osmosis is a spontaneous process, where water molecules move around randomly and cross the membrane in both directions without spending any external energy (Brownian movements). When a salt (osmotic agent) is dissolved in the water on one side of the membrane, each ion of a dissolved salt crystal attracts and binds several water molecules. The permeability of these hydrated ions through the membrane is much lower than for the free water molecules, and they stay on one side of the membrane due to a combination of their size and the complex hydrophilic/hydrophobic properties of the membrane. As the salt molecules bind a high number of water molecules, the concentration of free water molecules is lowered, resulting in a spontaneous net flow of water molecules from the freshwater side to the saltwater side of the membrane. The attraction between the ions and the water molecules further increases the net flow of water molecules through the membrane (Figure 2).
In the Subcuject WBI, the osmotic agent is released to a “pressure chamber” when the activation button is pushed. A few seconds after activation, the osmotic process starts and water is driven through the membrane to the pressure chamber. The excess water flow builds up a pressure and moves the plunger in a drug-filled cartridge, pushing the content of the cartridge out through the connected needle mechanism. The speed at which the plunger is driven is determined by several factors such as membrane characteristic/permeability and area, osmotic agent and concentration. A patented design ensures that the flow is constant over the entire injection and independent of the device orientation.
“An injection device is all about delivering a pharmaceutical product, often biologic, and this must be stable in the device during the shelf life of the product. Therefore, primary packaging is generally a major concern for pharmaceutical companies.”
ADVANTAGES OF USING AN OSMOTIC-DRIVE MECHANISM
From a user’s perspective there are several advantages associated with using an osmotic/hydraulic-driven device. One of the most apparent advantages is the size of the device. As the drive mechanism is hydraulic, there is no need for a mechanical plunger rod, which is often a primary factor for defining the size of an injection device. A 5 mL version of the WBI has approximate dimensions of 84 x 48 x 18.5 mm, while a 10 mL version of the device is anticipated to have dimensions around 93 x 51 x 20 mm – which is expected to be significantly smaller than most competitive wearable injectors in development.
The absence of mechanically moving parts has the added advantage that no sounds are made during injection and, except for “click” sounds during activation of the device and at retraction of the needle, the device is completely soundless. Furthermore, as pressure needs to be built up before the plunger starts moving, there is a soft injection start for increased patient comfort and minimised risk of impact damage in the primary container.
As the device contains only a small number of parts, a non-toxic salt and no electronic parts, the device is as acceptable for disposal as a single-use autoinjector.
From the perspective of a pharmaceutical company, the use of inexpensive elements and the fact that no electronical components need to be handled during development, assembly, storage and disposal of the device is advantageous. As previously noted, the cost of the WBI is expected to be comparable with an autoinjector and, therefore, offers a considerably more cost-effective solution than most competitive prefilled wearable injectors.
Osmotic processes can be very strong and easily create a pressure above 40 bars under the right conditions. The Subcuject WBI operates within an approximate pressure range of 0.2–1 bar, depending on the viscosity of the drug, and as this is in the lower pressure range of the osmotic process, the flow rate is relatively modestly affected by high viscosity. The flow rate when injecting a drug with a viscosity of 100 cP is just 25–30% lower than for a drug with a viscosity of 1 cP. Accumulated volume injection from the 3 mL device is shown in Figure 3 with typical examples of 1 cP and 100 cP fluids.
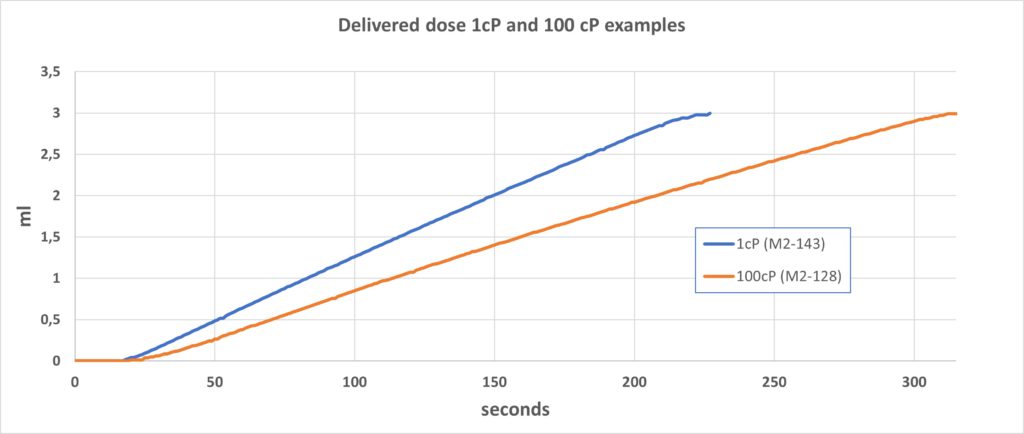
Figure 3: Delivered dose 1 cP and 100 cP examples.
Figure 4: Final assembly step –cartridge insertion.
An injection device is all about delivering a pharmaceutical product, often biologic, and this must be stable in the device during the shelf life of the product. Therefore, primary packaging is generally a major concern for pharmaceutical companies. Most drugs are tested in glass and with standard rubber compounds during early stability testing and, as such, selecting a device using such preferred components and materials poses a low risk of experiencing drug stability issues. The Subcuject WBI is based on a glass cartridge and a plunger made of a standard rubber compound.
In manufacturing, the drug-filled cartridge is assembled as the last part and can be added to the device by the pharma company or CMO (Figure 4).
OUTLOOK
Subcuject has previously demonstrated a 3 mL wearable injector and is now working with Phillips-Medisize on a 5 mL device that will be ready for demonstration during 2021. The forward osmosis principle used in the Subcuject WBI is not limited to injection of certain volumes, and larger volume devices are planned for development.
Phillips-Medisize and Subcuject are ready for engagement into drug-specific development programmes with pharmaceutical companies.

