To Issue 164
Citation: Bürdel S, Abedian R, “The Importance of User-Centric Design When Developing Devices for Home Use.” ONdrugDelivery, Issue 164 (Sep 2024), pp 36–40.
Simon Bürdel and Reza Abedian discuss the importance and value of conducting human factors studies and user consultations above and beyond regulatory requirements when designing user-centric devices for subcutaneous self-injection in at-home settings.
“Ensuring that patients can correctly and safely self-administer SC injections is a critical challenge, as errors could lead to under-dosing or decreased adherence, which may impact a therapy’s effectiveness.”
In recent years, the healthcare and life science industries have seen a change in how biologics are commonly administered. Therapeutics that were previously solely administered via the intravenous (IV) route in a clinical setting are now also being developed for subcutaneous (SC) administration at home. This transition is being driven by the potential for cost savings, shorter injection times, reduced burden on healthcare providers (HCPs) and increased convenience for patients.1–3 In fact, many leading pharmaceutical companies are responding to this shift in preference and developing IV and SC formulations in parallel, rather than seeking approval for the IV formulation first. However, ensuring that patients can correctly and safely self-administer SC injections is a critical challenge, as errors could lead to under dosing or decreased adherence, which may impact a therapy’s effectiveness.4
Organisations developing devices for self-administration must, of course, prioritise safety, performance and regulatory compliance. However, in order to deliver an optimal device that meets the needs of users, pharma companies, healthcare systems, supply chain members and the environment, there are many other factors to consider, including:
- Ease of use
- Storage
- Place of use
- Instructions for use (IFU)
- Connectivity
- Packaging
- Sustainability.
Developing product solutions that are user-centric and respond to the diverse needs and pain points of various stakeholders will therefore require extensive research and direct input and feedback from users.
GOING BEYOND REGULATORY REQUIREMENTS
According to Donald A. Norman, a prominent expert in cognitive science and advocate for user-centric design, “We need to build products that work the way people expect them to work”. This adage applies well to the pharmaceutical industry. If the development of a medical device is driven by technology alone, one might bring a functional device to market that performs well. However, without iterative user testing, the resulting product may not fully address the needs and expectations of patients. This may negatively impact all stakeholders and lead to potential use-errors. To ensure that medical devices address the correct needs and pain points of all potential users, it is necessary to consult not only patients but also lay caregivers, nurses and physicians.
To ensure safe use, US FDA Guidelines and IEC Standards demand a risk-based human factors (HF) and usability engineering approach. Manufacturers must identify critical tasks, develop and implement risk mitigations, and carry out iterative formative evaluations and a final summative evaluation. The product will be deemed safe and effective for use once all the relevant evidence has been generated and documented.5–7
This process, which is referred to as usability engineering, is comprised of four stages:
- User research via interviews and surveys to identify specific user needs
- Analysis, identification and categorisation of hazard-related use scenarios
- Design and implementation of a user interface and completion of formative evaluations
- Summative evaluation, which is the final usability validation of a device.
In addition, applying a comprehensive user-centric design approach not only relies on data collection and observation, but also seeks qualitative feedback on the user experience and subjective opinions of a presented solution, which goes beyond technical and regulatory requirements.
PUTTING USERS FIRST: VALIDATE AND INTEGRATE PATIENT INSIGHTS
In response to its continuous monitoring of user needs and market trends, Gerresheimer initiated a development project for an on-body SC device for delivery of large-molecule drugs. The predominant factors that influenced this decision were:
- A progressive shift from IV to SC injection as a preferred route of administration
- The potential for supporting patient independence through home therapy delivery
- The growth of biopharmaceuticals in product pipelines
- An increasing focus on sustainability from end users and the pharmaceutical industry.
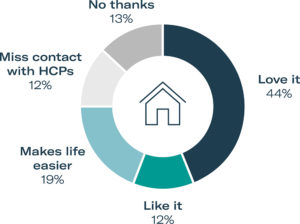
Figure 1: Opinion of at-home administration from PPI study participants.
In order to validate these market trends and user needs, Gerresheimer began by gathering patient preference information (PPI). Such user input collected at a very early stage of device development provides valuable insights into user needs. Gerresheimer focused its PPI study on administration of immunotherapy with monoclonal antibodies for oncology care. Patients and HCPs completed online surveys and in-person interviews. Overall, 75% of 32 US patient participants had a positive view of at-home SC administration, compared with in-clinic IV administration. Alongside the positive responses to at-home administration, researchers also received feedback from 12% of patients that they would prefer to go to a clinic, as they felt that they would miss the contact with their HCP, and another 13% who would refuse to receive their therapy at home (Figure 1).
In addition, 68% of patients had a positive opinion of an on-body SC injection device compared with a standard manual injection by a syringe carried out in a clinical setting. Moreover, HCPs provided similar responses – they reported that less time may be required to prepare for SC injections, which could potentially free up more time for patient consultation.
“Negative feedback is equally valuable and, in this instance, these patients made a critical point in oncology care, patients often develop a close relationship with their care team, particularly as side effects in immunotherapy can be varied.”
In research, it can be tempting to look past the patients who say “no”, especially if they represent a small percentage of study participants. However, negative feedback is equally valuable and, in this instance, these patients made a critical point – in oncology care, patients often develop a close relationship with their care team, particularly as side effects in immunotherapy can be varied. This patient feedback emphasised the potential for digital health solutions to facilitate connection between patients and healthcare teams and support symptom management. Gerresheimer’s development team therefore incorporated connectivity into its large-molecule device concept.
ADVANCING BIOPHARMACEUTICAL DELIVERY WITH USER-CENTRIC, SUSTAINABLE DESIGN
Following the positive outcome of the PPI study, Gerresheimer moved ahead with the development of an on-body SC device platform for biopharmaceuticals. The resulting cartridge-based system, Gx SensAir®, handles volumes of up to 10 mL as standard and viscosities of up to 50 cP. Beyond the functional and safety requirements for delivering sensitive biologics, two other principles guided the concept design – user-centricity and sustainability.
In response to these principles, the Gx SensAir® is partially reusable, featuring a two-part design with a reusable electronics unit and a sterilised disposable unit containing the needle. A prefilled cartridge with the medicinal drug product is separately refrigerated and inserted into the disposable unit before the two parts are clicked together. This design can reduce total treatment costs, waste and carbon footprint by minimising shipping, sterilisation and cold storage requirements (Figure 2).
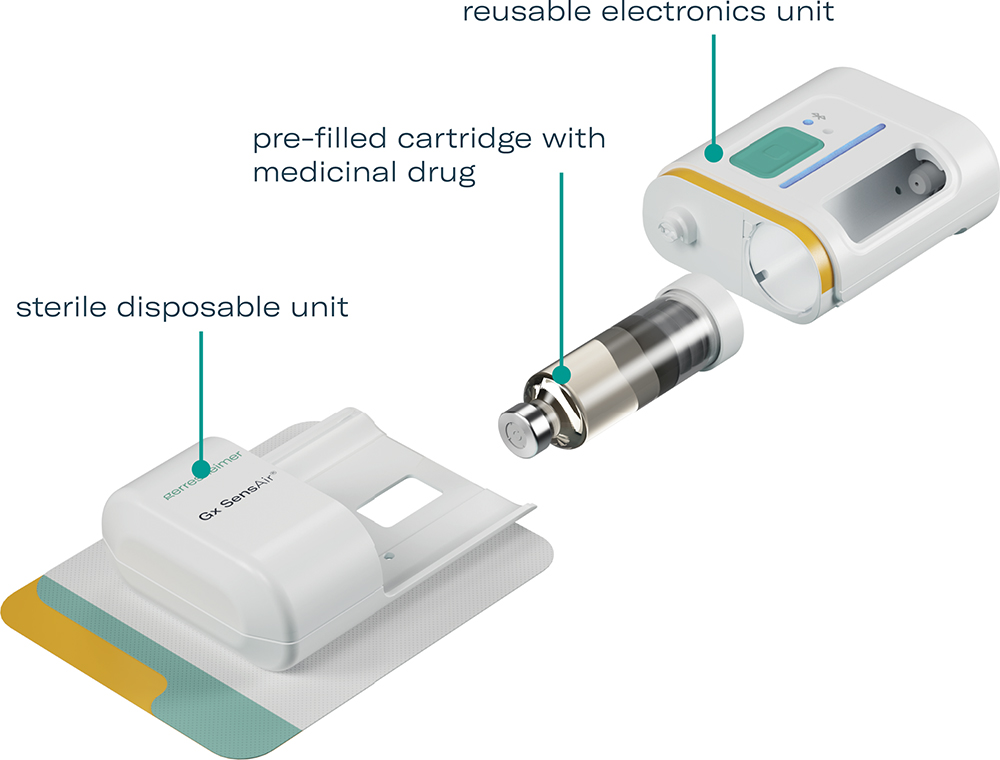
Figure 2: The modular, sustainable design of the Gx SensAir® on-body drug delivery device for biologics.
To address the patient treatment experience, the Gx SensAir® includes simple one-button operation, a light indicator that leads the user through the therapy step by step and an observation window for monitoring injection progress. The reusable unit also incorporates Bluetooth connectivity for data transfer and the possibility to connect to a digital health system, such as the Aptar Digital Health software platform for oncology.8
“As the sustainable two-part design concept for the Gx SensAir® requires some user assembly, it was imperative to confirm that all potential users could effectively assemble the device and apply it onto the body.”
HOW USER-CENTRIC DESIGN INFORMS PRODUCT DESIGN
Identifying and addressing potential issues early in the product development process through an HF study allows for modifications to be made, re-tested and further refined in subsequent studies. As the sustainable two-part design concept for the Gx SensAir® requires some user assembly, it was imperative to confirm that all potential users could effectively assemble the device and apply it onto the body. It was also important to ensure that this sustainable concept was well accepted.
Human Factors Study One
The first formative study with 10 participants concluded in January 2023, at Gerresheimer’s Advanced Technologies division in Olten, Switzerland. The primary objective of this study was to evaluate the device’s user interface (UI) to discover unanticipated use errors, test and optimise the user interface and discover potential foreseeable misuse cases for the device.
Device set-up, administration and disposal of the Gx SensAir® were tested with healthy lay users. Five key potential use errors were observed. Design mitigations were implemented directly into the development of a second prototype to optimise device railings, cartridge insertion, injection feedback LEDs, liner removal and the release mechanism. The feedback from this study was critical, as it enabled Gerresheimer to further optimise the device design to make it even safer, easier and more effective for users.
Human Factors Study Two
With the design mitigations from the first study implemented, another formative study was conducted in February 2024 in London, UK, with 30 participants. The scope was extended to the Gx SensAir® system and UI, including storage, device set-up, administration and disposal. The sessions were structured with simulated use testing, root cause analysis (RCA), knowledge-based assessment (KBA), a second RCA, subjective feedback gathering, conclusion and debriefing. In total, four different user groups were tested:
- Eight adult patients with multiple sclerosis (MS)
- Seven HCPs with experience in treating patients with MS
- Seven adult oncology patients with HER2 positive breast cancer
- Eight HCPs with experience in treating oncology patients.
The patient cohorts were split into injection-experienced and injection-naïve. The patients tested the device on an injection belt, while the HCP cohort carried out the administration on a mannequin. The results confirmed that design modifications implemented following the previous study effectively mitigated the identified use errors and difficulties (Figure 3).

Figure 3: Optimisation of usability confirmed by HF study participants.
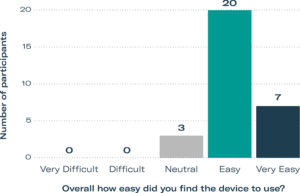
Figure 4: HF study participants gave positive ratings for ease of use.
During the subjective feedback session, participants were asked to rate their satisfaction and ease of use of the device on a five-point Likert scale. 67% of participants rated usage as “Easy”, and an additional 23% rated the device as “Very Easy” to use (Figure 4), with the remaining 10% as “Neutral”. Some participants who rated the ease of use as Easy or Neutral attributed their responses to the fact that it was their first time using the device. The results also showed that participants appreciated the device, with 53% stating that they were “Satisfied” and the remaining 47% stating that they were “Very Satisfied”.
Participants were asked to give their feedback on their preference for receiving therapy at home or in the hospital. 28 out of 30 participants (93%) said they would prefer to take their therapy at home. The reasons that participants gave for home injections being preferable included:
- Waiting times in hospitals
- Travel to the hospital
- Convenience
- Need for childcare
- Reducing pressure on the UK NHS.
HCPs stated that at-home injections would give patients more independence and freedom. Some stated that it would reduce pressure on hospitals, which could reduce waiting times and help solve capacity issues. Some HCPs also pointed to the risk of acquiring infections in hospitals and the potential impact of this on patients with chronic conditions (Figure 5).
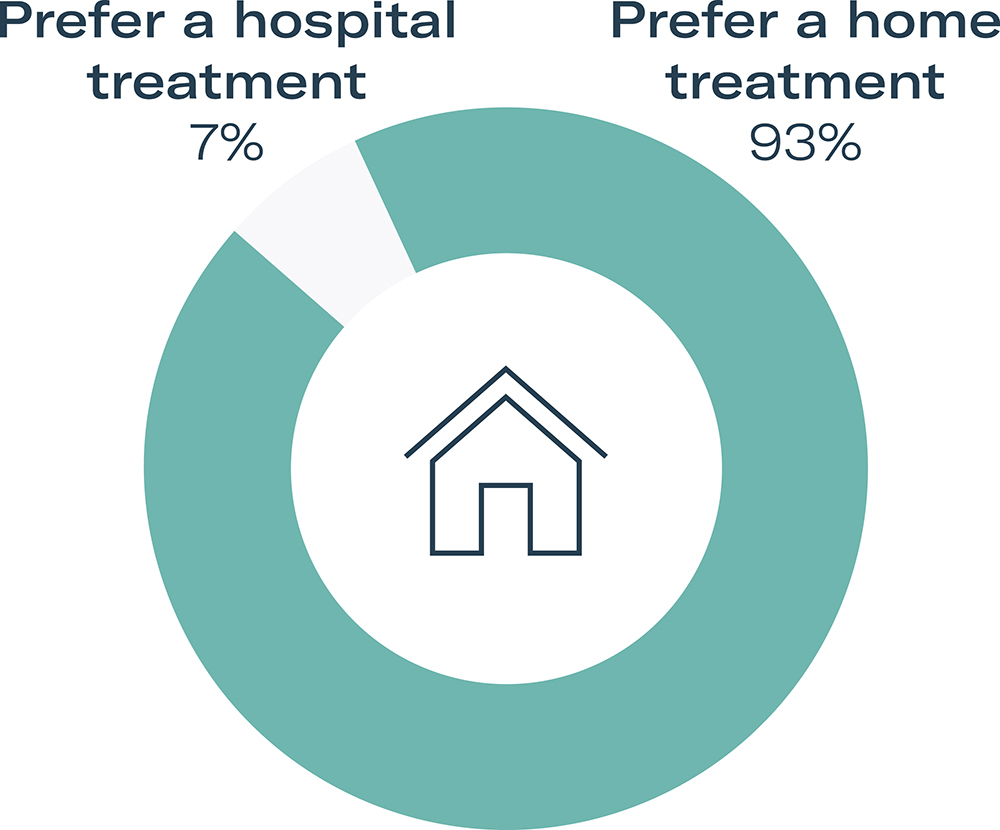
Figure 5: Study participant preference for at-home care versus treatment in a hospital or clinic.
Participants were also asked to give their feedback on sustainability and their preference for a reusable or single-use device. 28 out of 30 participants (93%) overwhelmingly stated a preference for a reusable device. Most of participants stated this was due to the environmental impact of single-use devices and that they would want to reduce waste and plastic. No participant stated a preference for single-use devices.
SUPPORTING PATIENTS IN THEIR EVERYDAY LIVES
As outlined in this article, user feedback is critical in order to ensure that a medical device for at-home use not only meets fundamental safety and performance requirements but also supports quality of life for patients with chronic conditions. Carrying out online surveys and contextual inquiries in the early product development phase can help confirm requirements identified from market monitoring and evaluation. Further feedback gathered through iterative formative evaluations, in the form of usability studies, can reconfirm the original conclusions and ensure that the device meets user expectations and needs.
“The studies also suggest that sustainability awareness is a big topic for the industry, as both patients and HCPs stated a preference for a reusable device over a disposable one, even when this involves additional user steps.”
These needs can vary according to the indication of a disease, as well as the mental, cognitive and dexterity abilities of the patient, so it is crucial that more than one user group is consulted. As discussed here, the studies conducted by Gerresheimer relating to the Gx SensAir® on-body device have shown that most patients and HCPs prefer that patients are able to administer their medication at home. The studies also suggest that sustainability awareness is a big topic for the industry, as both patients and HCPs stated a preference for a reusable device over a disposable one, even when this involves additional user steps.
Gerresheimer’s aim is to innovate and deliver for a better life every day. The evolution of the Gx SensAir® on-body device for self-administration illustrates the value of user input to fulfil this mission. It also highlights that negative feedback is as important as positive, as it helps ensure that devices meet the needs and expectations of all users in order to improve patients’ quality of life.
REFERENCES
- Dychter SS, Gold DA, Haller MF, “Subcutaneous drug delivery: a route to increased safety, patient satisfaction, and reduced costs”. J Infus Nurs, 2012, Vol 35(3), pp 154–160.
- Bittner B, Richter W, Schimdt J, “Subcutaneous Administration of Biotherapeutics: An Overview of Current Challenges and Opportunities”. BioDrugs, 2018, Vol 35(5), pp 425–440.
- Tetteh EK, Morris S, “Evaluating the administration costs of biologic drugs: development of a cost algorithm”. Health Econ Rev, 2014, Vol 4(1), article 26.
- Tjalma W, Huizing MT, Papadimitriou K, “The smooth and bumpy road of trastuzumab administration: from intravenous (IV) in a hospital to subcutaneous (SC) at home”. Facts Views Vis Obgyn, 2017, Vol 9(1), pp 51–55.
- “IEC 62366-1:2015: Medical devices – Part 1: Application of usability engineering to medical devices”. IEC, 2015.
- “Guidance for Industry: Applying Human Factors and Usability Engineering to Medical Devices”. FDA, 2016.
- “ANSI/AAMI HE75:2009 (R2018) Human factors engineering – Design of medical devices”. ANSI/AAMI, 2018.
- McKeon D, Yoon S, “Bringing Digital Health and Drug Delivery Together to Support Subcutaneous Injection in Oncology”. ONdrugDelivery, Issue 162 (Jun 2024), pp 10–14.

