Citation: Davison Z, Rose C, “The Pivotal Role Played by Device Developers to Improve Patient Eye Care. ONdrugDelivery, Issue 118 (Apr 2021), pp 12–16.
Zoë Davidson and Carolyn Rose discuss the challenges glaucoma and dry eye patients face with eye drop administration and present the Novelia® system featuring patented PureFlow® technology, which controls the medication flow.
INCREASING PREVALENCE OF CHRONIC EYE DISEASE
“Controlling the number of drops that are delivered was the most cited complaint from both dry eye and glaucoma patients. Patients noted more than one drop may come out at a time or even leaked a bit before being squeezed. This becomes even more problematic when the bottle is almost empty, as patients must squeeze harder to get the drop(s) out and feel they have even less control.”
Dry eye disease is among the most common diseases in ophthalmology, with a prevalence of between 5% and 34%. Studies from specialist centres have found higher prevalence of up to 57%. Dry eye leads to more frequent patient-doctor contact than glaucoma.1
Glaucoma is a chronic, progressive optic neuropathy and a leading cause of blindness. In 2020, it was estimated that approximately 80 million people have glaucoma worldwide, and this number is expected to increase to over 111 million by 2040.2 An increase in teleworking as a result of covid-19 has been a contributing factor to an increase in “digital eye strain”. In addition, Google search activity analysis using Google Trends revealed that “sore eyes” were reported as the most significant ocular symptom of covid-19, with a significant spike in the number of Google searches over the last 10 months.
“Our eyes were not designed to use computers, especially for long periods of time – and, as a result, many people who spend long hours reading or working on a computer experience eye discomfort and vision problems,” said Barbara L Horn, OD, President of the American Optometric Association.3
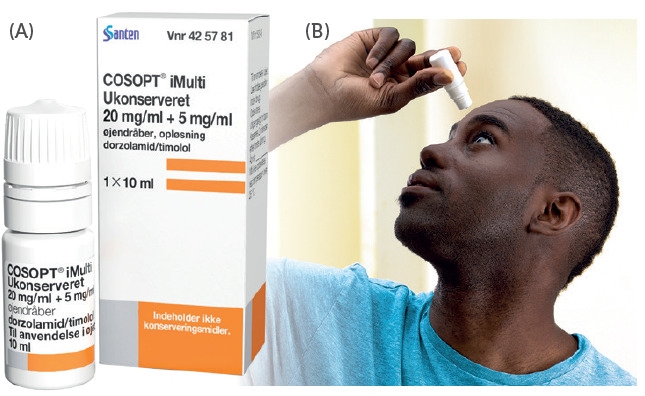
Figure 1: (A) Cosopt iMulti® for the treatment of intraocular pressure in patients with open-angle glaucoma using Novelia®, marketed by Santen (Image courtesy Santen). (B) 76% of patients prefer Novelia® preservative-free multi-dose eyedropper.
Novelia®, Nemera’s preservative free multidose eyedropper, has over 200 references on the market for prescription and over-the-counter (OTC) use, with 86% of these products dedicated to dry eye and glaucoma treatments (Figure 1A). A study was conducted by Nemera’s R&D department, Insight Innovation Centre, in August 2020 to understand challenges encountered by dry eye and glaucoma patients with their current medication. A total of 16 participants were included in the study. This group included patients under treatment for dry eye and glaucoma, ophthalmologists and nurse practitioners forming the healthcare professional (HCP) sample. The participants were asked to briefly discuss their background and experience as it related to managing their/their patients’ eye condition including symptoms, current therapies and treatment practices, as well as relevant challenges or frustrations.
GLAUCOMA VERSUS DRY EYE SYNDROME (REGIMENTED VERSUS REACTIVE)
While the patients involved in Nemera’s study did not experience daily symptoms, glaucoma patients were still very aware that medication non-compliance can lead to vision loss, which motivates them to take their medication regularly. It has been found that the most important factor in the management of glaucoma patients is educating them about the disease of glaucoma itself.4 Failure to acknowledge local symptoms and underplaying them as subjective disturbance can be highly detrimental.1
Glaucoma patients have set regimens as prescribed by their provider and have commonly fit this regimen into their morning/nightly routine. Thus, very few reported forgetting to take their medication or struggling to remember to do so. Note that Nemera’s sample had “simple” regimens (e.g. 1–2 drops in the morning and/or at night), but physicians noted regimens can become more complex (multiple types of drops, 3–4 times/day). Additionally, some patients may use OTC dry eye drops, as irritation can be a side effect of some glaucoma medications. According to a 2008 survey conducted in Germany, over 50% of all glaucoma patients have dry eye. In addition, dry eye was more common if three or more anti-glaucoma agents were used as well as with increasing duration of glaucoma, suggesting preservatives have an influence on the development of dry eye.5
The hypovolaemic dry eye is manifested clinically as symptoms of a feeling of dryness, a feeling of a grain of sand or foreign body in the eye, and stinging. The eyes often feel tired and symptoms typically only occur during the day during stress.1
Contrary to glaucoma sufferers, dry eye patients manage their condition as needed. They will experience dry eye symptoms (i.e. irritation, dryness, a “scratchy feeling”) usually as a result of an activity (e.g. extended computer/screen time, wearing contact lenses, etc) or their environment (e.g. wind, seasonal allergies, etc).
Most patients will take drops (1–4 at a time) as needed throughout the day when they start to experience symptoms. Because of this, they typically carry drops with them (in a bag or pocket) throughout the day and/or store bottles in different locations where they might need them (e.g. at work or in the car).
More severe dry eye sufferers may take a drop first thing in the morning or at night as recommended by their provider, or use a prescription-grade medication.
PATIENT CHALLENGES DURING DROP ADMINISTRATION
During the study, patients commonly cited two key challenges relating to eye drop administration, the first of which being lack of control.
Controlling the number of drops that are delivered was the most cited complaint from both dry eye and glaucoma patients. Patients noted more than one drop may come out at a time or even leaked a bit before being squeezed. This becomes even more problematic when the bottle is almost empty, as patients must squeeze harder to get the drop(s) out and feel they have even less control.
While a minor annoyance to dry eye patients, this is more impactful for glaucoma patients who are unsure if they have received the correct dose and/or are concerned about running out of their expensive medication prematurely.
Another challenge cited by patients was the uncertainty around remaining drops. Many eye dropper bottles on the market today are opaque and those that are not are predominately covered by the label. This makes it difficult to see how much remains. And, even if you are able to see, it is difficult to know how many doses/drops that volume equates to. Many patients described shaking the bottle to “guesstimate” how many doses remain.
Dry eye patients can typically purchase another OTC bottle if they run out, but for glaucoma patients, whose refills are regulated by insurance (US-based study), there can be uncertainty and concern regarding the amount remaining (particularly if multiple drops had unwittingly been administered and/or refills are not automated).
LIMITING FACTORS FROM THE HCP PERSPECTIVE
“Novelia’s patented PureFlow® technology not only serves as a venting system but also controls the medication flow. Nemera has adapted the flow control within Novelia® that avoids multiple drop delivery into the eye and ensures that only one calibrated drop is dispensed at a time.”
Self-administration can be challenging for patients with arthritis, tremors and/or hand strength/dexterity issues that can make removing the cap, gripping and squeezing the bottle, and holding the hand steady during administration challenging.
Additionally, as described by physicians, there is a subset of patients who do not do well putting things into their eyes. These patients may require aiming techniques and ways to hold the eyelid steady (to avoid blinking). In a worst-case scenario, these limitations may preclude self-administration, requiring a caregiver.
Compliance was also noted as a challenge by all HCPs, who commonly noted this becomes more of an issue with more complex regimens. Nearly nine out of 10 glaucoma patients are unable to instil eye drops correctly and therefore an easy-to-use system that is appreciated by patients could contribute to improving their compliance with a treatment.6 One HCP who participated in the study stated that: “When you have one drop, compliance is 75–80%. When you use two drops, it drops to 65%. If three drops, it drops below 50%”.7
Additionally, patients (especially those with cognitive issues) may not always remember or follow storage and administration instructions such as refrigerating the medication or shaking before use.
PATIENT EYE CARE MANAGEMENT AND HOW NOVELIA® OFFERS A SOLUTION
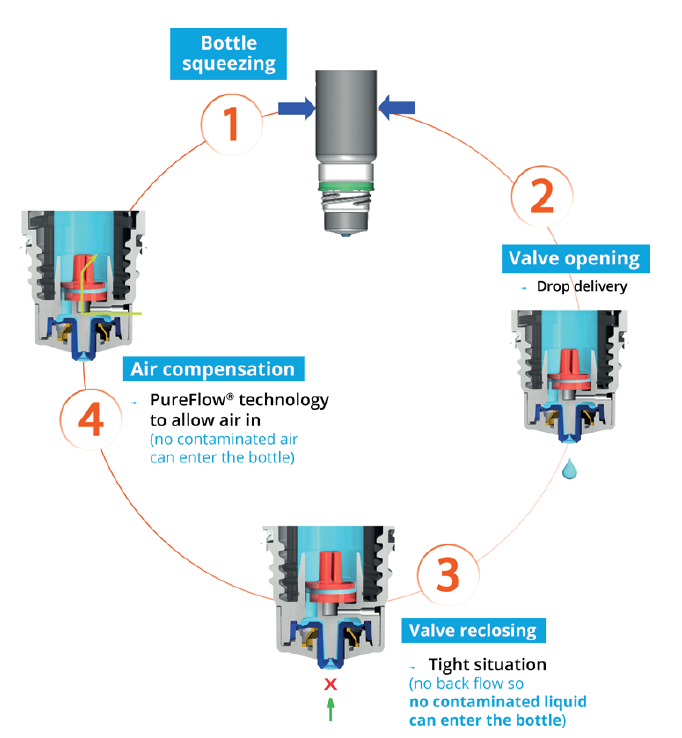
Figure 2: The Novelia® system uses a non-return valve that removes the need to filter the liquid, making it possible to use a silicone membrane to filter the air.
Control
Patients and HCPs alike desire a bottle that allows users to control the number of drops delivered, consistently delivering a single drop with each actuation.
Novelia’s patented PureFlow® technology not only serves as a venting system but also controls the medication flow (Figure 2). Nemera has adapted the flow control within Novelia® that avoids multiple drop delivery into the eye and ensures that only one calibrated drop is dispensed at a time. Nemera offers three different PureFlow® versions, each tailored to formulations of differing viscosities, from highly liquid to highly viscous. In addition, five different valve sizes are available, each one delivering a different calibrated drop size. This allows Nemera to customise the drop size depending on specific product requirements. This improved control leads to increased patient confidence (of accurate dosing), and reduced frustration and medication waste.
Ease of Use
Users, even those with dexterity issues and tremors/shaking, must be able to effectively handle and manipulate the delivery system and administer a drop.
Contributing factors to Novelia® being the preservative-free multidose eyedropper preferred by 76% of patients included the intuitiveness of the screw-on cap and the associated reassurance and squeeze force required towards the end of the product’s life. Novelia® required only 6% more pressure to squeeze the bottle from the beginning to the end of the treatment, compared with 35% for the other device (Figure 3).8
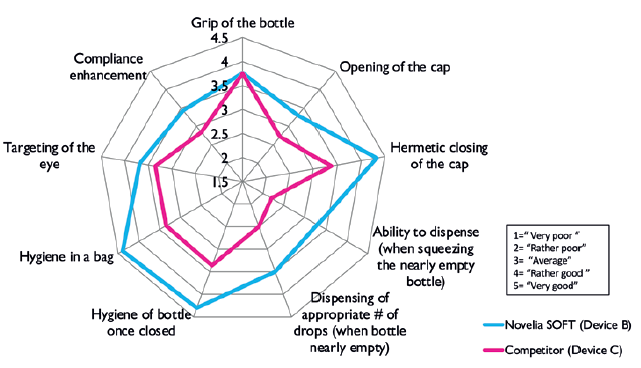
Figure 3: Mean scores across different parameters given by 90 patients with
glaucoma, dry eye or conjunctivitis using Novelia® and another marketed device.
Novelia’s patented blue tip is also a favourite feature of the device. It helps patients target the eye before drop administration and anticipate the angle of the drop on to the ocular surface.
Transparency
Patients need more visibility of their medication supply, so they can replenish as needed and do not find themselves without.
A full range of bottles is available in terms of size, material and sterilisation type (5 mL, 7.5 mL, 11 mL and 15 mL). All sizes are available in low-density polyethylene either in white or natural (transparent), allowing patients to know when their medication is running low. Nemera is also developing polypropylene and cyclic olefin copolymer bottles for specific formulation compatibility. Novelia® has been validated using both gamma and ethylene oxide sterilisation.
Portability
A user must be able to transport their medication easily. Note that for dry eye patients this is likely to be daily, while for glaucoma patients this is likely only for overnight/travel.
The Novelia® device features a screw-on cap that fits tightly on to the device nozzle, which is optimal in terms of portability. Patients have found other marketed devices comprising a snap-on cap to be less robust, with several instances of leaking during transport, be it in a handbag or pocket. As such, Novelia® outperformed another marketed device in terms of cap opening, hermetic sealing and nomadic use, with a mean score of 4.5 out of five for the three features.9
WHAT IS NEMERA PUTTING IN PLACE TO CONTINUE TO SERVE CUSTOMERS IN THE OPHTHALMIC SPACE?
To serve customers in supporting patient needs, Nemera has recently extended its manufacturing capabilities and, in doing so, has doubled its capacity to produce the Novelia® preservative-free multidose eyedropper (Figure 4).

Figure 4: Nemera’s headquarters in La Verpillière, France, has doubled its capacity to produce the Novelia® preservative-free multidose eyedropper.
In addition to Nemera’s continued investment to support the production side, Nemera is equally reinforcing its offering in services. Nemera firmly believes that developing a holistic and comprehensive patient experience and human factors management strategy can create significant competitive advantages and, ultimately, result in safe, effective and differentiated combination products that respond to patient needs.
Nemera continues to offer a range of laboratory services, including testing of customers’ bulk formulation, allowing Nemera to determine the best Novelia® configuration for a formulation. Nemera can recommend the most suitable PureFlow® control, bottle type and valve size to achieve the desired drop calibration.
Nemera can also assist customers in finding the right ready-to-go dossier available for private labelling of certain molecules with the Novelia® device, and has a substantial list of partners, formulation licensors and fillers, all working in collaboration to bring customers a finished drug device combination with Novelia®.
In conclusion, Nemera’s unwavering commitment to address patient needs and translate them into meaningful solutions truly differentiates the combination products offered across its delivery routes, including ophthalmology.
REFERENCES
- Erb C, “Glaucoma and Dry Eye”, 2020, 2nd ed, Bremen: UNI-MED.
- Tham Y et al, “Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and metaanalysis”. Ophthalmology, 2014, Vol 121(11), pp 208–290.
- Forster V, “Are Your Eyes Hurting During The Coronavirus Pandemic? You May Have “Computer Vision Syndrome””. Forbes.com, June 2020.
- Feng A et al, “Success of patient training in improving proficiency of eyedrop administration among various ophthalmic patient populations”. Clin Ophthal, 2016, Vol 10, pp 1505–1511.
- Erb C, Gast U, Schremmer D, “German register for glaucoma patients with dry eye. I. Basic outcome with respect to dry eye”. Graefes Arch Clin Exp Ophthalmol, 2008, Vol 246(11), pp 1593–1601.
- Gupta R et al, “Evaluating eyedrop instillation technique in glaucoma patients”. J Glaucoma, 2012, Vol 21(3), pp 189–192.
- “User study performed by Insight Innovation Centre”. ICH report, 2020.
- “User study performed for Nemera by GfK to understand the Novelia® market opportunities versus competitors”. GfK report, 2015.
- “User study performed for Nemera by QUALIQUANTICI, Novelia® and a marketed pump product testing”. Marketing Espace report, 2018.

