To Issue 170
Citation: Mulpas J, “The Power of Pulmonary: Advances in Inhaled Drug Delivery Expand Respiratory Treatment Options”. ONdrugDelivery, Issue 170 (Apr 2025), pp 8–12.
Jonathan Mulpas discusses how advanced inhaled drug delivery devices, such as dry powder inhalers and non-propellant liquid inhalers, can meet currently unmet needs in the inhalation space, focusing on devices that Aptar and its partners are developing to realise this potential and the expertise that Aptar can offer in this area.
Recent advances in inhaled drug delivery technology are making inhalation a viable option for a wide range of new drugs. Historically, most inhaled products required low but frequent doses of a drug, targeted respiratory indications such as asthma or chronic obstructive pulmonary disease (COPD) and were administered from a pressurised metered dose inhaler (pMDI). Since their introduction to the market, pMDIs have proven to be a reliable and well-established vehicle to treat these common conditions.
“FROM CYSTIC FIBROSIS TO TUBERCULOSIS AND A RANGE OF INFECTIOUS DISEASES, THE LATEST GENERATION OF ADVANCED INHALED DRUG DELIVERY TECHNOLOGIES CAN PROVIDE NUMEROUS BENEFITS TO DRUGMAKERS AND PATIENTS ALIKE.”
However, the advent of newer advanced inhaled drug delivery technologies, such as dry powder inhalers (DPIs) and non-propellant liquid inhalers (NPLIs), has brought new capabilities and distinct advantages that are proving to be game changers in the inhaled drug delivery space. These advanced inhalation technologies not only retain the reliability and convenience of traditional pMDIs but can also provide highly controlled, targeted delivery and high dose loading for molecules and target indications not previously possible. From cystic fibrosis to tuberculosis and a range of infectious diseases, the latest generation of advanced inhaled drug delivery technologies can provide numerous benefits to drugmakers and patients alike.
CATEGORIES OF INHALATION DRUG DELIVERY TECHNOLOGY
Portable inhalation drug delivery technologies can be grouped into three main categories:
- pMDIs: Low-dose drug delivery in a suspension or solution with a propellant
- DPIs: Low- or high-dose powder inhalation
- NPLIs: Inhaled fine mist liquid dispensers for targeted delivery.
This article focuses on the latest advances in DPI and NPLI technology – how they enable drugmakers to meet currently unmet needs and deliver a wide range of new molecules and indications via inhalation. Aptar Pharma has added advanced inhaled drug delivery technology to its well-established portfolio of pMDI metering valves and has incorporated advanced inhalation technologies as part of its strategy to provide a full range of inhaled drug delivery solutions.
DPI VERSUS pMDI TECHNOLOGY
Having the ability to deliver dry powder formulations directly to the lungs can be an attractive option for a drugmaker. Dry powder formulations do not include any aqueous media and can therefore provide enhanced stability in a lightweight format, which is ideal for portability and extended shelf life.
On the other hand, pMDIs represent a well-accepted and proven drug delivery technology that uses a propellant to deliver fine droplets of drug formulation through the mouth and throat to the targeted lung area. They have long been used to effectively deliver low doses of drug product to respiratory patients for chronic conditions such as asthma and COPD. However, as traditional pMDI propellants contribute to the product’s CO2 footprint, Aptar is in the process of redeveloping its industry-standard pMDI metering valves to work with the latest lower-global-warming-potential propellants for greater sustainability.
Going forward, pMDIs are expected to remain a staple and proven performer for low-dose inhaled delivery. However, pMDIs do not provide the level of flexibility offered by advanced DPIs, which are capable of delivering high doses of a range of different molecule types, including biologics, directly to the lungs.
ADVANCED DPI ADVANTAGES
Advanced DPI technology now enables drugmakers to administer high doses of API directly to a patient’s lungs while avoiding their cough reflex, significantly increasing the number of potential applications for this non-invasive drug delivery route. Inhaled drug delivery not only provides targeted lung delivery but can also enhance the drug’s bioavailability and provide rapid onset of action for faster symptom relief.
“pMDIS DO NOT PROVIDE THE LEVEL OF FLEXIBILITY OFFERED BY ADVANCED DPIS, WHICH ARE CAPABLE OF DELIVERING HIGH DOSES OF A RANGE OF DIFFERENT MOLECULE TYPES, INCLUDING BIOLOGICS, DIRECTLY TO THE LUNGS.”
By delivering the API directly to the lungs, inhaled delivery can result in a reduced side effect profile by avoiding the higher doses of drug typically required for systemic drug delivery approaches. The most advanced DPI technologies are able to provide enhanced dosing precision, even in the face of highly variable patient lung function. In light of this, Aptar Pharma has developed solutions or partnered with leading inhaled drug delivery technology developers to offer some of the most advanced DPI and NPLI options available on the market.

Figure 1: The Quattrii DPI is capable of delivering carrier-based or API-only
low-potency formulations of 50–150 mg fill mass.
QUATTRII DPI
Aptar Pharma is exclusively commercialising the Quattrii DPI (Figure 1) through a collaboration with Cambridge Healthcare Innovations. The Quattrii DPI system can precisely deliver up to 100 mg of powder formulation to the lungs via its unique tunable vortex effect, created within the inhalation system. Able to separate formulation carriers from the product blend through classification, Quattrii creates a vortex in the blister upon inhalation that sweeps respirable-sized particles into the inhalation airflow while leaving the carrier particles behind. This process efficiently transports the powder deep into the lungs where it can be directly absorbed into the lung tissues. Alternatively, for engineered particles, the system can be tuned to deliver all of the powder formulation, without separation, directly to the lungs of the patient.
The Quattrii DPI reduces dose-to-dose variability, which is caused by differences in patient inhalation capabilities. Even when the patient has poor lung function, Quattrii efficiently transfers available inhalation energy to the powder formulation, which in turn aerosolises it and creates a consistent fine particle fraction for reliable dosing. This precise dosing also minimises unwanted mouth and throat deposition, instead focusing the delivery on the lungs. With a high payload capability of 100 mg of powder formulation, the Quattrii DPI system can now be used for a wider array of targeted lung treatments including antivirals, antifungals, antibiotics, anti-hypertensives and even cystic fibrosis drugs.

Figure 2: Orbital can reliably deliver high doses of dry powder formulations directly to the lungs.
APTAR ORBITAL® DPI
Aptar’s Orbital® DPI (Figure 2) was designed to provide progressive delivery of very high payload powder drug formulations to the lungs. Orbital’s unique powder delivery system centres around a deagglomeration chamber where a puck composed of powder drug formulation is progressively released as the patient inhales through the rate-controlling orifices that access the chamber. As air is drawn through the peripheral inlets, powered by patient inhalation, the jets of air propel the powder puck in an orbital motion around the chamber, deagglomerating and releasing the desired amount of drug product with each inhalation.
The result is that the inhalation-driven air jets drive the spinning puck to release the correct amount of drug powder, which is then carried through the mouth and throat for delivery to the lungs. The patient simply inhales from the device repeatedly until the entire puck has been completely deagglomerated to ensure that the full dose has been delivered. Each inhalation can be progressive, releasing only a partial dose, which reduces the risk of activating the patient cough reflex, which can contribute to increased patient compliance and consistent dosing.
Some conventional DPI technologies require the manual loading of multiple individual powder-filled gelatine capsules into the inhaler system one at a time to deliver a high dose. These complex manual loading systems can contribute to higher rates of dosing errors and reduced patient compliance. Conversely, the Orbital DPI uses a unique progressive dispensing system from a single drug formulation puck that eliminates the need to reload the device when delivering a high load dose (Figure 3).
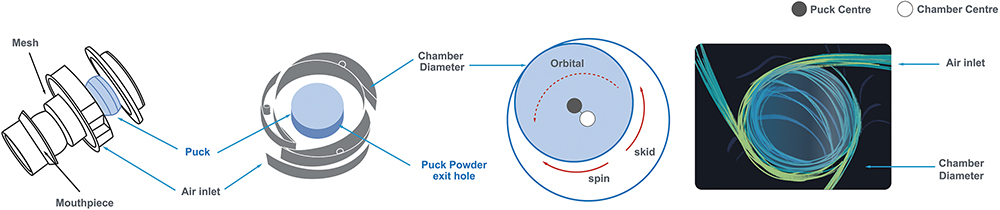
Figure 3: Description of the Orbital chamber, powder release and deagglomeration mechanism.
“ORBITAL IS EASY TO USE, REQUIRING ONLY A SIMPLE PRIME AND BREATH STEP, RESULTING IN SHORT ADMINISTRATION TIMES.”
The flexibility of the Orbital DPI allows it to be used for single- or multiuse applications. The Orbital system can be reused by simply loading a new puck into the DPI system every time a dose is required. Orbital pucks come in sealed airtight blister packaging that protects the drug product from air or contaminants until it is required for use, further enhancing the shelf life of the product. Orbital is easy to use, requiring only a simple prime and breath step, resulting in short administration times. Orbital is also compatible with awide range of formulations and molecule types and can reliably deliver 100 mg to over 400 mg of formulation from each powder puck. With scalable solutions from Phase I to commercial, the high payload capabilities of the Orbital DPI expand the number and types of APIs that can be administered via this safe and convenient drug delivery route.
KOLIBRI NPLI
The Kolibri digital NPLI is the result of an exclusive collaboration between PULMOTREE and Aptar Pharma, designed to fulfil the unmet needs of inhaled drug delivery when using a fine mist – many of the currently available liquid inhaler technologies cannot achieve sufficient control of the aerosol deposition pattern to reliably target the deeper parts of the lungs.
Many older liquid inhalation technologies cannot compensate for the varied inhalation capabilities of different patients or variations in the inhalation manoeuvres they carry out. These factors can result in inconsistent and uncontrolled aerosol deposition. Other traditional nebulisers are non-portable and require that the patient remains stationary for extended periods of time to deliver a low-concentration liquid formulation to the lungs.

Figure 4: Kolibri, a digital mesh nebuliser system for inhaled drug delivery.
On the other hand, the Kolibri NPLI (Figure 4) is highly portable and provides new features combined with levels of performance that can enhance the patient experience. With the incorporation of the Intelligent Administration System (iNAS), Kolibri provides visual and haptic feedback to the patient regarding their inhalation manoeuvres, effectively training them on how to optimise their inhalation technique. This real-time guidance allows Kolibri to provide much more efficient targeted delivery to the desired areas of the lungs for optimal drug delivery, while enhancing efficacy and reducing drug loss (Figure 5).
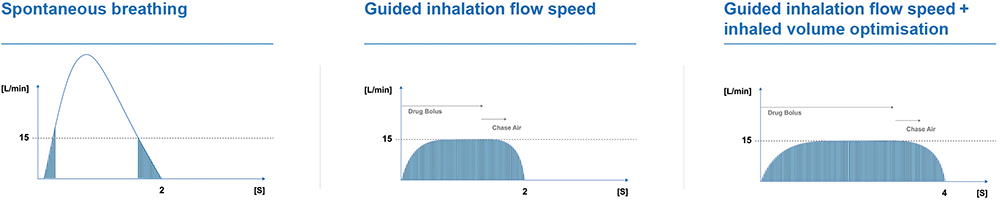
Figure 5: Kolibri’s unique drug deposition optimisation through inspiratory flow control.
The Kolibri NPLI is an advanced soft mist drug delivery system that incorporates integrated digital health connectivity, designed to resolve many of the limitations of existing nebuliser-like inhalation devices. Packed with innovative new features, the Kolibri vibrating-mesh nebuliser is triggered by the user’s breath. Kolibri’s performance can be optimised for the requirements of specific medications, patient groups or even market strategies.
Device data regarding inhalation technique, inspiratory flow, inhaled volume, time, date and aerosol generator life meter can be captured and automatically uploaded to the cloud for review and analysis almost anywhere. This makes Kolibri ideal for use in clinical studies or for self-administered home use. Physicians or researchers can conveniently monitor patient usage and determine the patient’s compliance from a distance. When connected to the Kolibri Connect smartphone app, patients can stay up to date with their dosing schedule through automated reminders and also review their user data via a single convenient interface. The proprietary key-and-lock system ensures that the device operates to its designed level of performance by locking out the use of non-genuine or expired aerosol heads for patient safety and the elimination of commercial product piracy risks.
“AS A LEADER IN THE INHALATION DRUG DELIVERY SPACE, APTAR PHARMA OFFERS ITS CUSTOMERS SPECIALISED DEVELOPMENT SERVICES THAT CAN RELIABLY SUPPORT THE CREATION OF COMPLIANT AND EFFECTIVE INHALED DRUG PRODUCTS.”
APTAR’S INHALATION DEVELOPMENT EXPERTISE
Successfully developing optimised drug delivery products for inhalation takes not only high-quality devices, but also specialised scientific and regulatory knowledge with supporting services. The path from device to lungs for liquid mists or powders is complex, with substantial differences between every patient and product. As a leader in the inhalation drug delivery space, Aptar Pharma offers its customers specialised development services that can reliably support the creation of compliant and effective inhaled drug products.
Aptar Pharma’s Nanopharm offers comprehensive orally inhaled and nasal drug product development services that can optimise formulations for each customer molecule and support drug delivery system selection. The company’s proprietary SmartTrack™ platform uses clinically relevant in vitro tests and in silico models, such as computational fluid dynamics, to support the acceleration of the development process, in some cases eliminating the need for Phase I clinical studies.
Aptar Pharma’s regulatory experts also provide comprehensive regulatory process support to customers, particularly for complex drug-device combination products, including the provision of a host of fully supportable filing-ready data sets. Furthermore, Aptar Pharma’s Noble group is available to provide medical device training solutions, human factors engineering, market insight services and patient onboarding strategies, simplifying the path to market for those developing innovative inhalation drug delivery products.
Aptar Pharma offers its customers a fully integrated service to bring advanced inhaled drug delivery products to market supported by its global product supply capabilities. Aptar Pharma has a proven track record of successfully supporting a wide variety of customer products, with over 150 US FDA approved NDAs, ANDAs and INDs using Aptar Pharma drug delivery technologies since 2020 alone. Aptar Pharma also protects its product innovations with over 750 patent families for enhanced intellectual property protection and market differentiation.
CONCLUSION
Aptar Pharma has invested in growing its portfolio of advanced inhaled drug delivery technologies because it sees an opportunity to safely and effectively treat more respiratory conditions via this non-invasive and reliable delivery route. With advances in new inhalation devices, the digitalisation of drug delivery and the creation of specialised drug development and support services, the future opportunities for inhaled drug delivery products will continue to grow. Aptar Pharma prides itself on delivering comprehensive services along the drug development pathway that help to take its customers through the journey from formulation to patient.
To find our more about Aptar Pharma’s inhalation offering, click here.

