To Issue 167
Citation: Parry M, McLaughlin J, Otte T, “Trace Analytical Considerations when Reformulating pMDIS with Next-Generation Low-GWP Propellant Systems”. ONdrugDelivery, Issue 167 (Nov 2024), pp 48–52.
Mark Parry, John McLaughlin and Tino Otte discuss the necessary studies required to understand the nitrosamine formation and leachables profiles of new pMDI propellants with lower global warming potentials as part of the pharmaceutical industry’s drive to reduce its emissions and environmental impact.
INTRODUCTION
Driven by political, commercial and regulatory action, reducing the carbon footprint of pharmaceutical products has become a critical focus for the whole industry. Within the respiratory space, the continuing evolution of the Montréal Protocols are focusing this work on switching from current pressurised metered dose inhaler (pMDI) propellants to new propellants with lower global warming potential (GWP).
This change presents challenges for both new pMDI products and the reformulation of existing products into the new propellants. Low-GWP propellants come with new chemistries that mean the reformulation of existing projects may not be as simple as a drop-in replacement. Interactions between formulation and device parts are well understood for hydrofluoroalkane (HFA) 134a and HFA-227a but present an evolving picture for the low-GWP alternatives, meaning that a review of the analytical methodology and overall chemistry, manufacturing and controls approach is needed. For reformulation, a suitable gap analysis of methods will be required, as these methods will underpin the generation of critical data supporting product characterisation, stability and in vitro bioequivalence work. This also presents opportunities to address key analytical issues associated with contaminants such as nitrosamines and leachables, which represent significant risks to patient safety and have been the focus of significant legislative development in recent years.
NITROSAMINES
Nitrosamine Formation in pMDIs
Since the EMA and US FDA introduced guidelines in 2020 on controlling nitrosamine impurities in medicinal products, industry stakeholders have undertaken extensive risk assessment and testing programmes. These efforts are time-consuming and costly, requiring the development of sensitive detection methods and comprehensive root-cause analysis. This has been an important step in improving patient safety but has led to significant reformulation work for some products to comply with new intake limits.
“The transition to low-GWP propellants in pMDIs provides a chance to proactively address nitrosamine formation early in formulation development.”
The transition to low-GWP propellants in pMDIs provides a chance to proactively address nitrosamine formation early in formulation development. Using advanced analytical techniques, such as liquid chromatography mass spectrometry (LC-MS) and gas chromatography mass spectrometry (GC-MS), can help streamline efforts to meet regulatory requirements for nitrosamine levels. Tandem LC-MS provides high sensitivity and specificity for detecting API-specific nitrosamines, even in complex pMDI matrices.GC-MS is effective for analysing volatile, non-API-specific nitrosamines and potential degradation products from propellants. Using both LC-MS and GC-MS in early trials enables comprehensive screening, identifies nitrosamine sources and ensures regulatory compliance.
Nitrosamines primarily form through reactions between secondary or tertiary amines and nitrosating agents, such as nitrous oxides or nitrites, under acidic conditions. In pMDIs, nitrosamine formation can result from interactions between the propellant system, APIs, excipients and device materials. The introduction of low-GWP propellants to pMDIs necessitates a thorough analytical evaluation to manage the risk of nitrosamine formation.
Next-generation low-GWP propellants, such as hydrofluoroolefin (HFO) 1234ze and HFA-152a, differ chemically from traditional propellants. As unsaturated compounds, HFOs can potentially react with other formulation components or degrade over time, forming reactive species that may contribute to nitrosamine formation. The interactions between these new propellants and other formulation components, especially in the presence of moisture, heat and light, are not yet fully understood.
Each component of the pMDI formulation, including APIs, excipients and device materials, must be evaluated for their potential to contribute to nitrosamine formation. Therefore, reformulation efforts should include rigorous testing with robust and validated methods at all stages of development to assess any increased risk when using these new propellants.
Environmental and Storage Considerations
The conditions under which pMDIs are stored and used (temperature, humidity and exposure to light) can significantly influence nitrosamine formation and should be carefully controlled. Accelerated stability studies can help predict how formulations might behave over time and under different environmental conditions, providing essential data for formulation decisions.
The new low-GWP propellant systems could mean changes in recommended long-term storage conditions or packaging to provide the reformulated product with the necessary stability to remain a viable commercial product.
“Reformulating pMDIs with low-GWP propellants enables the risk of nitrosamine formation to be addressed early in the drug development process, something that wasn’t considered when many of the currently marketed pMDI products were being developed in the 1990s and 2000s.”
Analytical Strategies and Regulatory Compliance
Reformulating pMDIs with low-GWP propellants enables the risk of nitrosamine formation to be addressed early in the drug development process, something that wasn’t considered when many of the currently marketed pMDI products were being developed in the 1990s and 2000s. Analytical and manufacturing strategies must align with regulatory guidelines and include robust risk assessments that consider all potential sources of nitrosamine contamination, including the supply chain, raw materials and manufacturing processes.
EXTRACTABLES AND LEACHABLES
The different chemical and physical properties of the new low-GWP propellants will mean that adjustments to existing formulations, and different drug and excipient combinations and inhaler designs may be necessary to account for the different characteristics of these propellants. Due to their new production processes, a unique impurity profile is to be expected. These impurities must be tested for their concentration and toxicity before the new propellants are used in clinical or commercial products.
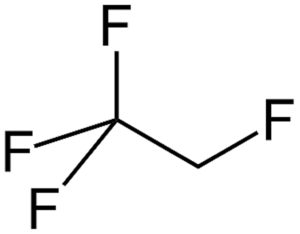
Figure 1: Structure of HFA-134a.
The physical and structural properties, such as density, vapour pressure, polarity and viscosity (Figures 1–3 and Table 1),1 undoubtedly affect the extraction behaviour and interaction with contact materials or storage containers, resulting in different profiles of extractables and leachables after long-term storage in pMDIs.
In a recent publication from Faucard et al, three fluorinated low-GWP propellants, HFO-1234ze, HFA-152a and HFA-134a, were compared regarding their leaching behaviour.2 In the study, a typical pMDI valve, consisting of polybutylene terephthalate (PBT), ethylene propylene diene monomer (EPDM) and COC (cyclic olefin copolymer), was exposed to these propellants and tested after intervals of zero, one, three and six months in storage with different analytical screening techniques. The leaching of target compounds was investigated with multiple analytical techniques, such as gas chromatography flame ionisation detection (GC-FID) and liquid chromatography with ultraviolet detection (LC-UV).
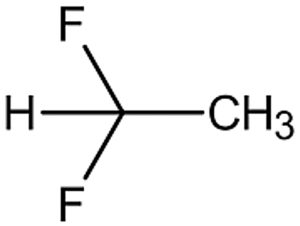
Figure 2: Structure of HFA-152a.
The PBT dimer and trimer was investigated as a representative compound for a non-volatile leachable. HFA-152a showed the largest increase in leaching of the dimer after six months, and the leaching of the trimer was also higher compared with the other blowing agents, although generally at a much lower level.
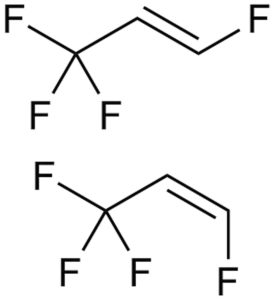
Figure 3: Structure of HFO-1234ze.
As representatives for the semi-volatile leachables, the “antioxidants” and “other semi-volatile leachables” were investigated and reported as individual compound-groups. Tetrahydrofuran (THF) was chosen as a representative example of a highly volatile leachable. The total concentration of leached species was below the toxicological limits, but there were differences reported in the leaching behaviour between the individual propellants.
Both the extractable concentration of the individual substance groups and the speed of extraction appear to depend strongly on the structures and physical properties of the gas molecules. Looking at the properties in Table 1, the different structure is also associated with differences in the physical characteristics.
| Propellant | Global Warming Potential (GWP) |
Vapour Pressure at 20°C (kPa) |
Surface tension at 20°C (mN/m) |
Density at 20°C (g/mL) |
Viscosity at 20°C (cP) |
Dipole momen at 20°C (debye) |
| HFA-134a | 1300 | 572 | 8.9 | 1.23 | 0.20 | 2.06 |
| HFA-152a | 124 | 510 | 10.4 | 0.91 | 0.24 | 2.30 |
| HFO-1234ze | <1 | 499 | 8.6 | 1.17 | 0.19 | 1.44 |
Table 1: Physicochemical characteristics of propellants used for the formulation of pMDIs.1
HFA-152a shows a higher extractability of the non-volatiles and THF as highly volatile, whereas, for the antioxidants and other semi-volatiles, HFA-152a and HFO-1234ze showed a slightly stronger and partially faster extraction behaviour, which indicates that more parameters than just the volatility of the leachable compounds play a role in the leaching behaviour. Unfortunately, only sum parameters or specific target compounds were investigated in this study, which prevents a correct correlation of the leaching behaviour with different molecular structures of the leachable compounds. There could be an increased selectivity of the new propellants, which would lead to a pronounced leaching of compounds with special structures.
In a pre-study, a rubber material component used as an inhaler valve gasket was analysed by thermodesorption (TDS)-GC/MS. TDS-GC/MS is a powerful technique that is often used for extactables profiling.3 As the thermal desorption approach does not involve a solvent and trapping of compounds, it covers multiple compound classes that could be thermally desorbed, including volatiles and many semi-volatile species.
The trapping of desorbed compounds means that even species with very low concentration can be detected. Table 2 shows a list of the compounds detected and identified in the rubber seal. Many different compounds from various substance classes were detected, such as hexane, a residual solvent; butylated hydroxytoluene, a stabiliser; partially halogenated rubber oligomers, a byproduct of rubber production; and diethyl phthalate, a plasticiser. It is clear that the rubber gasket contains many small molecules of varying structure and polarity, all of which could be selectively leached out by formulations that include the new low-GWP propellants.
| Compound |
| n-Hexane |
| Hexanal |
| 1-Bromohexane |
| 2,6-Dimethyloctane |
| 2,2,4,6,6-Pentamethylheptane |
| 2-Octanone |
| Octanone |
| Limonene |
| p-(1-Propenyl)-toluene |
| Nonanal |
| 1,3,3-Trimethyl-2-(2-methylcyclopropyl)-1-cyclohexene |
| 4a-Methyldecahydro-2H-benzo[a]cyclohepten-2-one |
| Rubber oligomer with sum formula C13H24 |
| Decanal |
| 1,2-Dibutylcyclopentane |
| Undecanal |
| Rubber oligomer with sum formula C14H26 |
| Brominated rubber oligomer |
| 2,6-Di-tert-butyl-4-hydroxy-4-methylcyclohexa-2,5-dien-1-one |
| Rubber oligomer with sum formula C14H28 |
| 2,6-Di-tert-butylbenzoquinone |
| Rubber oligomer with sum formula C13H23Br |
| Butylated hydroxytoluene |
| Diethyl phthalate |
| Rubber oligomer with sum formula C13H23Br |
| 2-Bromo-4,6-di-tert-butylphenol |
| 3,5-Di-tert-butyl-4-hydroxybenzaldehyde |
| Rubber oligomer with sum formula C25H48 |
| Rubber oligomer with sum formula C21H39Br |
| Dibutylphthalate |
| Rubber oligomer with sum formula C21H39Br |
| 1-Hexadecanol |
| Bromo-alkane |
| Rubber oligomer with sum formula C25H48 |
Table 2: Compounds in a bromobutyl rubber material detected and identified by TDS-GC/MS, sorted according to increasing retention time.
As demonstrated in previous systematic studies, the extraction behaviour depends on the physical properties and structures of the new low-GWP propellants.2 Since pMDI systems, especially the valves, are typically composed of many different materials, all with unique additive sets and other low-molecular-weight impurities, there is some risk of leaching of new compounds or higher levels from the traditional materials used in pMDIs when low-GWP propellants are introduced.
“The unique extraction selectivity of the new fluorinated propellants must be investigated in more detail to understand the differences and to rule out a toxicity issue for dedicated compounds.”
The unique extraction selectivity of the new fluorinated propellants must be investigated in more detail to understand the differences and to rule out a toxicity issue for dedicated compounds. In the literature published so far, only a few targets or sum parameters have been investigated.2 The next step will be a dedicated generic extractables study that compares the extraction behaviour of traditional propellants (including HFA-227a) with the new low-GWP candidates in more detail. In particular, the effects on a wide range of extractable compounds with different structures and polarities needs to be investigated in order to assess the risk of pronounced leaching for certain compounds and the potential toxicity problem associated with this.
According to US Pharmacopeia <1663>, the extractables screening of individual construction materials is helpful to assess complex container closure systems, such as pMDI products. Techniques such as TDS are cited as the method of choice to detect a wide range of compounds with little effort, as direct analysis is possible without prior extraction. This material-specific extractables screening study should be important to trace the extractables detected after incubation of the combined pMDI device using quantitative screening techniques to characterise the extract, such as GC-MS and LC-MS, which need to be optimised to capture a wide range of different compounds.
As the extractables content in most plastics is typically very low, even a small variation in the additive, as would be expected in different batches of material, could distort the results, making it difficult to adequately compare the different propellants. For a comparative study on leachables, it will be important to compare the same type of inhaler, ideally from the same batches of material, incubated with the different types of propellants. The pure propellant gas should be used as the incubation medium to exclude variations in the drug formulation. Alternatively, a suitable control sample or blank sample of the formulation should be stored in an inert container under the same conditions and analysed using the same techniques to exclude effects not caused by the inhalation system.
After the quantitative evaluation of the results, the differences in extraction behaviour and extraction selectivity of the new low-GWP propellants, compared with the traditionally used propellants, should be investigated peak by peak to prove whether or not there are major differences for specific compounds, compound classes or whether the extraction behaviour is similar for a wider range of compounds and has a similar selectivity to the old solvents.
After the results of this more substance-specific extractables study are known, a general indication could be given if big differences in extraction behaviour should be expected for the new low-GWP propellants. As a best-case scenario, there may only be a small difference over all classes of extractables and no selective effect in specific plastic ingredients. In such a case, the traditional construction materials could be further used without concern.
“The transition to next-generation low-GWP propellants in pMDIs is a crucial step towards reducing the environmental impact of respiratory treatments.”
CONCLUSION
The transition to next-generation low-GWP propellants in pMDIs is a crucial step towards reducing the environmental impact of respiratory treatments. With this comes new analytical challenges in ensuring product safety, particularly concerning nitrosamine formation and the presence of leachables. A comprehensive analytical approach is necessary to understand and mitigate these risks. By optimising formulation components and employing advanced analytical techniques, the pharmaceutical industry can successfully reformulate pMDIs with low-GWP propellants while ensuring patient safety and compliance with regulatory standards.
REFERENCES
- Buttini F et al, “Metered dose inhalers in the transition to low GWP propellants: what we know and what is missing to make it happen”. Expert Opin Drug Deliv, 2023, Vol 20(8), pp 1131–1143.
- Faucard P et al, “Leachables Assessment from a New Generation of pMDIs using Low Global Warming Potential Propellants and the Impact of Ethanol Filling”. Resp Drug Deliv, 2024, Vol 1, pp 194–197.
- Scherer N, “Leachable and Extractable Studies on Single-Use System Technologies in commercial scale Drug Filling Lines”. Dissertation, LMU München, Aug 2019.

