Citation: Houston A, Sachs M, Rolland H, “Unlocking the Potential of Intranasal Drug Delivery”. ONdrugDelivery, Issue 154 (Nov 2023), pp 58–62.
Allan Houston, Marcel Sachs and Heiko Rolland discuss the healthcare potential of intranasal drug delivery systems and the necessary steps for bringing these solutions to market.
“Intranasal delivery is one of the most effective ways to treat opioid overdoses by providing a rapid and easily accessible route
of administration for life-saving medications, such as naloxone.”

Figure 1: Silgan Dispensing’s Monodose Nasal System.
Silgan Dispensing’s newest healthcare solution, the Monodose Nasal System (Figure 1), is an intranasal solution that gives healthcare professionals a simpler, faster method for administering medicine directly to the patient. The Monodose Nasal System is a primeless, ready-to-use device that can be used with one hand from any direction, due to its 360° delivery capability. The solution also is optimised for bioequivalence to help pharmaceutical partners expedite product commercialisation.
Increasingly, intranasal solutions, such as the Monodose Nasal System, have been shown to be effective at administering drugs such as naloxone, which counters the potentially lethal effects of an opioid overdose. The single-dose system allows emergency and healthcare professionals to quickly deliver these life-saving interventions.
THE POTENTIAL OF INTRANASAL DRUG DELIVERY SYSTEMS
What Are Some of the Applications?
Intranasal delivery solutions are an excellent alternative to other administration methods – namely oral and parenteral applications. Medications taken orally must first pass through the digestive system, which reduces their concentration and, subsequently, makes them slower to take effect.
Intravenously administered drugs take effect much faster than orally administered ones, but can make many people anxious, plus they require medical training to use. Intranasally administered drugs can be rapidly diffused to the brain, avoiding the digestive system and bypassing the blood-brain barrier (BBB), which can be an obstacle for larger drug molecules. Furthermore, intranasal devices can be used by people without medical training and do not carry the stigma associated with needles.
What Are Some of the Use Cases?
Intranasal delivery systems are poised to significantly impact a variety of markets, especially opioid overdoses and migraines. Intranasal delivery is one of the most effective ways to treat opioid overdoses by providing a rapid and easily accessible route of administration for life-saving medications, such as naloxone. In the US alone, there were nearly 80,000 opioid-involved overdose deaths last year – a 60% increase in just three years (Figure 2). As such, the global naloxone spray market is expected to grow accordingly. The life-saving and financial potential for a much simpler, more effective way to treat opioid overdoses cannot be overstated.
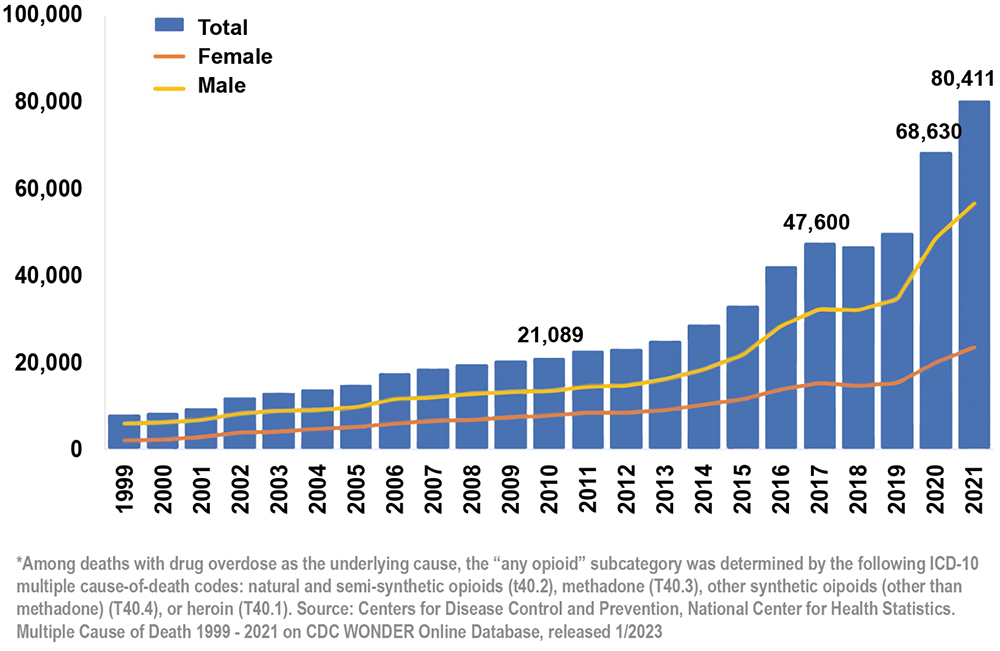
Figure 2: National overdose deaths involving any opioid – number among all ages by gender, 1999–2021.
Migraines are another health concern that could benefit from a single-dose intranasal delivery device. Beyond the intense headaches, migraines can cause vomiting, sensitivity to light and sound and even temporary vision loss. More than one in 10 people worldwide experience migraines, which can be extremely debilitating.
While opioid overdose and migraine headache applications make up a significant portion of the potential market, there are many other indications that can benefit from Silgan Dispensing’s Monodose Nasal System. Subject to clinical testing, these indications could potentially include treating epileptic seizures, anaphylaxis, chronic cancer pain, treatment-resistant depression and severe hypoglycaemic episodes, to name but a few.
Furthermore, intranasal delivery systems continue to be a popular method for vaccine administration, especially among children and the elderly. This is understandable, as children are frequently wary of needles, which can make critical vaccine appointments strenuous for everyone involved. With older patients, many have less stable veins, which can make successfully administering an intravenous treatment both difficult and painful. A simple nasal spray could ease these situations considerably.
“As a design and manufacturing partner, Silgan Dispensing offers industry-leading support for pharmaceutical partners seeking to get a new device to market in a timely fashion.”
BRINGING A NEW INTRANASAL DELIVERY DEVICE TO MARKET
Both drugs and their delivery devices share a similar market approval process, but delivery devices have a higher to-market success rate than drugs. While only roughly one in every 5,000 drugs that enter preclinical testing gets approval for therapeutic use – a process that takes 12 years, on average – delivery devices tend to be approved more frequently. However, that does not mean drug delivery devices have simple regulatory requirements. Getting caught up in regulatory missteps can lead to costly delays, revisions and resubmissions.
For a successful marketing authorisation application, a reliable drug delivery device manufacturer should be well-positioned to support a pharmaceutical manufacturer applicant. This requires being able to navigate the complex device and combination product regulations of different market requirements. Additionally, having state-of-the-art manufacturing processes for the delivery device in place will help strengthen the applicant’s regulatory submissions.
The compatibility of the device with the formulation should be investigated and established before going into further clinical phases, bioequivalence testing and stability studies. The design and performance of an intranasal delivery device will directly affect the clinical efficacy of the overall combination product.
The key considerations for an intranasal delivery device should include stability with the targeted formulation components, a user-friendly design, device reliability and dosing accuracy. Furthermore, the device’s material of construction should be carefully considered – critically, it must be compliant with regulatory and pharmacopoeial requirements, as well as have low extractables profile for the polymers, additives and metallic components of the device to ensure biocompatibility and patient safety.
HOW SILGAN DISPENSING AVOIDS COSTLY REGULATORY DELAYS
Providing a meaningful and approved package of supportive regulatory and technical data about the delivery device is essential for a marketing authorisation approval process. As a design and manufacturing partner, Silgan Dispensing offers industry-leading support for pharmaceutical partners seeking to get a new device to market in a timely fashion.
Unmatched Regulatory Expertise
Silgan Dispensing’s commitment to regulatory compliance and its collaboration with industry stakeholders are pivotal factors in avoiding costly regulatory delays. Its bioequivalence programme, meticulously designed to align with US FDA and EMA regulatory requirements, not only expedites drug delivery device development, but also instils confidence in its customers regarding healthcare packaging compliance.
Bioequivalence Programme Bioequivalence testing helps Silgan Dispensing’s customers overcome regulatory and technical barriers that have previously slowed product commercialisation (Figure 3). Solutions like its Monodose Nasal System and Gemini™ BE Nasal Pump showcase Silgan Dispensing’s ability to deliver expedited delivery device development that fits its customers’ needs.

Figure 3: Silgan Dispensing conducts testing for its bioequivalence programme in its Healthcare Centre of Excellence in Hemer, Germany.
Silgan Dispensing has also used its nasal spray testing cGMP-compliant lab and equipment and developed partnerships with accredited external cGMP labs and contract manufacturing organisations (CMOs) specialising in nasal products. This collaborative approach ensures that Silgan Dispensing’s customers receive full support and streamlined regulatory pathways, minimising the risk of obstacles and delays.
“Ergonomics is central to Silgan Dispensing’s design philosophy –
across all markets, its dispensing solutions must be comfortable
and have a user-friendly design.”
Extensive Drug Master Files
Silgan Dispensing also develops and submits extensive drug master files, including full biocompatibility data and reliability studies to ensure that the appropriate regulatory bodies, whether that be the FDA or EMA, are provided with the necessary information. Working closely with the different authorities and constantly being up to date is key to a quick and trouble-free qualification. This helps Silgan Dispensing’s customers get their products to market timely and efficiently.

Figure 4: ISO Class 7 cleanroom production and ISO Class 8 cleanroom warehouse in Silgan Dispensing’s Healthcare Centre of Excellence.
Dynamic Production Facilities
Silgan Dispensing’s Healthcare Centre of Excellence in Hemer, Germany, is compliant with the highest manufacturing standards and boasts a variety of certifications (Figure 4):
- ISO Class 7 cleanroom for products
- ISO Class 8 cleanroom for warehousing
- ISO quality system certifications for cGMP ISO 15378
- Medical device quality systems for ISO 13485
- Registered as an FDA medical device establishment.
THE CONNECTION BETWEEN ERGONOMICS AND MEDICATION ADHERENCE
Why is Ergonomics Important?
Ergonomics has been found to play an integral role in medication adherence. When ergonomics is factored into the device and packaging design, it evokes a positive user experience, leading to a higher percentage of medication adherence. Medications that prove challenging or inconvenient to use are unlikely to achieve the level of adherence necessary to realise their full therapeutic effect. This is why ergonomics is central to Silgan Dispensing’s design philosophy – across all markets, its dispensing solutions must be comfortable and have a user-friendly design.
The Monodose Nasal System is a notable example of this. While it is not the first intranasal delivery device on the market, the way it is designed improves upon other available options. Unlike others, the Monodose Nasal System incorporates textured concave finger pads to keep fingertips in place, optimising it for a more comfortable and secure one-handed actuation (Figure 5).

Figure 5: Enhanced ergonomics of Silgan Dispensing’s Monodose Nasal System.
How Does the Right Design Benefit Both Healthcare Brands and Patients?
A thoughtful design for a healthcare brand not only fosters trust and credibility among healthcare providers, but also plays a crucial role in managing healthcare costs. In the short term, patients feel more confident in administering their medication, which leads to increased adherence. Over the long term, this design approach aids effective condition management and leads to a healthier life.
Silgan Dispensing’s healthcare team is guided by a design philosophy centred on delivering the best user experience and enhancing adherence. This philosophy serves as the driving force behind the innovation and effectiveness of all its drug delivery devices.

