To Issue 160
Citation: Guillemot L-H, Brocco B, Pagnoud E, Sircoulomb P, “Vial Stopper Compatibility: Criteria and Testing Methods for Pharmaceutical Packaging”. ONdrugDelivery, Issue 160 (May 2024), pp 74–80.
Laure-Hélène Guillemot, Benjamin Brocco, Edouard Pagnoud and Pascal Sircoulomb, discuss some of the key parameters to consider when choosing a vial stopper and present evaluation methods that were developed to test and demonstrate the compatibility of various stoppers with commonly used vial designs.
In both the creation of new pharmaceuticals and the repurposing of existing medications, the role of packaging is crucial and should not be overlooked. A thorough understanding of packaging materials and their compatibility is fundamental to ensuring that medications are kept in an environment that safeguards their quality until usage is required.
The primary container, being in direct contact with the medication, is pivotal in preserving a medication’s quality from production, through packaging and distribution, to storage and eventually administration. Any issues with the primary container could lead to negative outcomes for patients – such issues not only have a profound human cost but could also lead to substantial financial losses for the manufacturer.
A critical aspect of the primary container’s role is to ensure the integrity of its closure throughout the medication’s lifecycle. For injectable drugs, which commonly use glass vials as the primary container, achieving this integrity hinges on the perfect compatibility between the vial and its rubber stopper.
“These challenges may manifest as difficulties in inserting stoppers, stoppers accidentally popping off prior to crimping or breaches in the CCI.”
Despite the standardisation of vial sizes, as outlined in ISO8362-1 for those made from glass cane and ISO8362-4 for moulded glass vials, compatibility challenges can emerge. These challenges may manifest as difficulties in inserting stoppers, stoppers accidentally popping off prior to crimping or breaches in the container closure integrity (CCI). Such issues not only complicate operational processes but may also pose a threat to patient safety.
To emphasise the importance of these risks, various vial neck designs have been created to specifically address pop-off issues – the European blow-back (EBB) and American blow-back (ABB) – that are derived from the standard non-blow-back (NBB) design. Choosing the appropriate combination of stopper and vial is therefore a crucial decision that influences both the packaging development and the efficiency of the drug fill/finish processes.
Aptar Pharma has honed its expertise over 50 years of collaborations with pharmaceutical and biotech companies, addressing packaging challenges for vaccines, biotech drugs and small molecules. Aptar Pharma’s vial stoppers leverage pure elastomer formulations and are designed with a holistic understanding of all compatibility issues that may be encountered when choosing a vial or stopper system.
“Optimising these parameters is key as a stopper that is too hard to insert may not be placed ideally on the vial, while a stopper that fits too loosely may accidentally fall off the vial, two situations that can put the integrity of the drug at risk.”
INSERTION FORCE
Controlling the force required to insert stoppers in a given vial can be key for ensuring operational efficiency and consistent performance on a filling line. This insertion force is influenced by several factors, such as the dimensions and designs of the stopper’s plug and the vial neck, the elastomer’s hardness and the quantity and nature of lubricant used on the stopper. Optimising these parameters is key as a stopper that is too hard to insert may not be placed ideally on the vial, while a stopper that fits too loosely may accidentally fall off the vial, two situations that can put the integrity of the drug at risk.
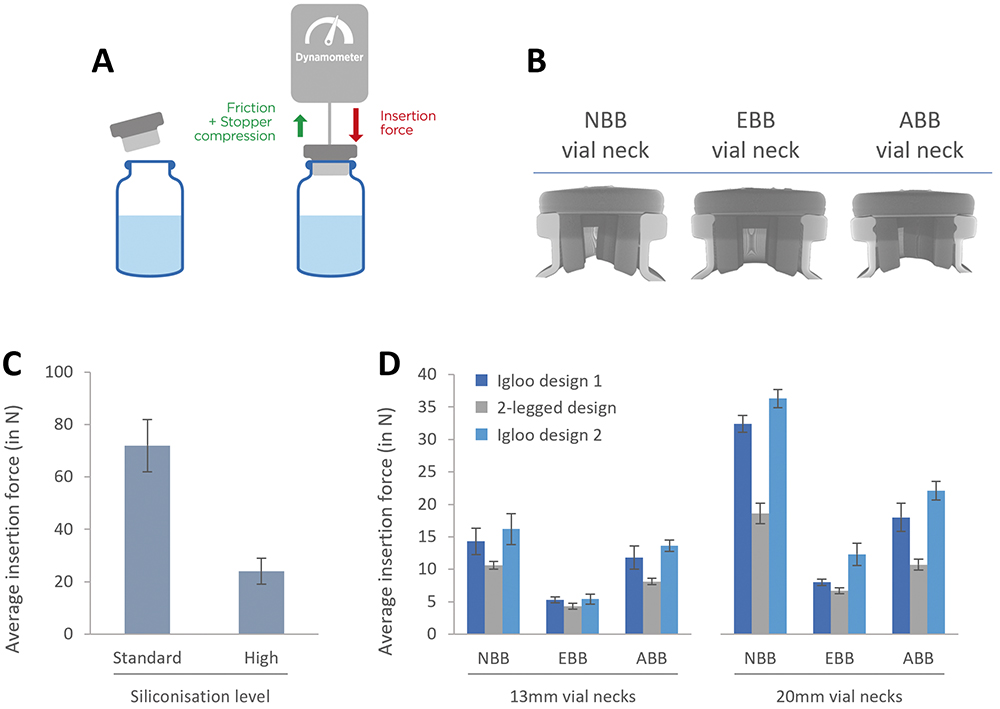
Figure 1: Evaluation of the insertion force with lyophilisation stoppers. A: Experimental set-up and representation of the forces that contribute to determining the insertion force. Stoppers are inserted at a constant speed of 100 m/min. B: X-ray tomography of an NBB stopper lyo two-legged design inserted in NBB, EBB and ABB vials. C: Evaluation of the impact of the siliconisation level on the insertion force. D: Evaluation of the insertion force for three different lyophilisation stopper designs with NBB, EBB and ABB vials.
To test these parameters, Aptar Pharma experts use an evaluation method that relies on the use of a dynamometer mounted on a test bench that inserts a given stopper on a given vial at a rate of 100 mm/min (Figure 1A). In these results, Aptar focused on evaluating the impact of siliconisation on the insertion force and tested three different stopper designs on three different vial neck designs. X-ray tomography was also used to provide insights in justifying the variation of insertion forces observed (Figure 1B).
Figure 1C compares stoppers that have been lubricated with different quantities of silicone oil, which is added during the drying step at Aptar Pharma. The data clearly show that the addition of extra silicone onto the stopper facilitates the insertion, reducing the insertion force by more than 60%.
Figure 1D shows the insertion force required to insert lyophilisation stoppers into various designs of 13 mm and 20 mm vials. When inserted into EBB vials, all three designs perform similarly. For both NBB and ABB vials, the two-legged design demonstrates a reduced insertion force compared with its igloo counterparts.
To further understand these results, X-ray tomography was performed, allowing a clear view of how the stopper is inserted in the vial neck. The position of the stopper in the vial neck seems to indicate that, with the EBB vial, the rubber is under less constraint, which could translate to lower insertion forces. For both the NBB and ABB vial designs, the legs of the stoppers are bent slightly inwards, indicating increased geometrical constraints, which could contribute to increasing insertion forces. In all three cases, the stoppers form a seamless seal with the vial’s flange and with the top of the neck.
These results demonstrate that the design of both the vial neck and the stopper can affect the insertion force for a vial/stopper combination. Furthermore, lubrication, in the form of silicone oil, can help dramatically modulate the insertion forces of a stopper by decreasing friction.
“The outcome – whether the stopper remains firmly seated or is forced out – depends on the design of the vial’s neck and the stopper, and how the rubber compresses against the glass.”
POP-OFF
When discussing the compatibility between a vial and a stopper, pop-off is one of the main issues that ought to be considered. “Pop-off” refers to the event when the vial stopper spontaneously dislodges itself from the vial neck in between the stoppering event and crimping the vial. This not only disrupts the filling process but also results in the loss of medication.
After a stopper is fitted into the neck of a vial, various forces are at play to determine whether or not it stays in place. Inserting the stopper increases pressure in the vial’s headspace, creating a force that could eject the stopper. Conversely, the friction between the rubber stopper and glass vial acts to keep the stopper secured. Therefore, the outcome – whether the stopper remains firmly seated or is forced out – depends on the design of the vial’s neck and the stopper, and how the rubber compresses against the glass. Insufficient forces holding the stopper, such as reduced friction due to excessive silicone lubrication, may lead to a pop-off.
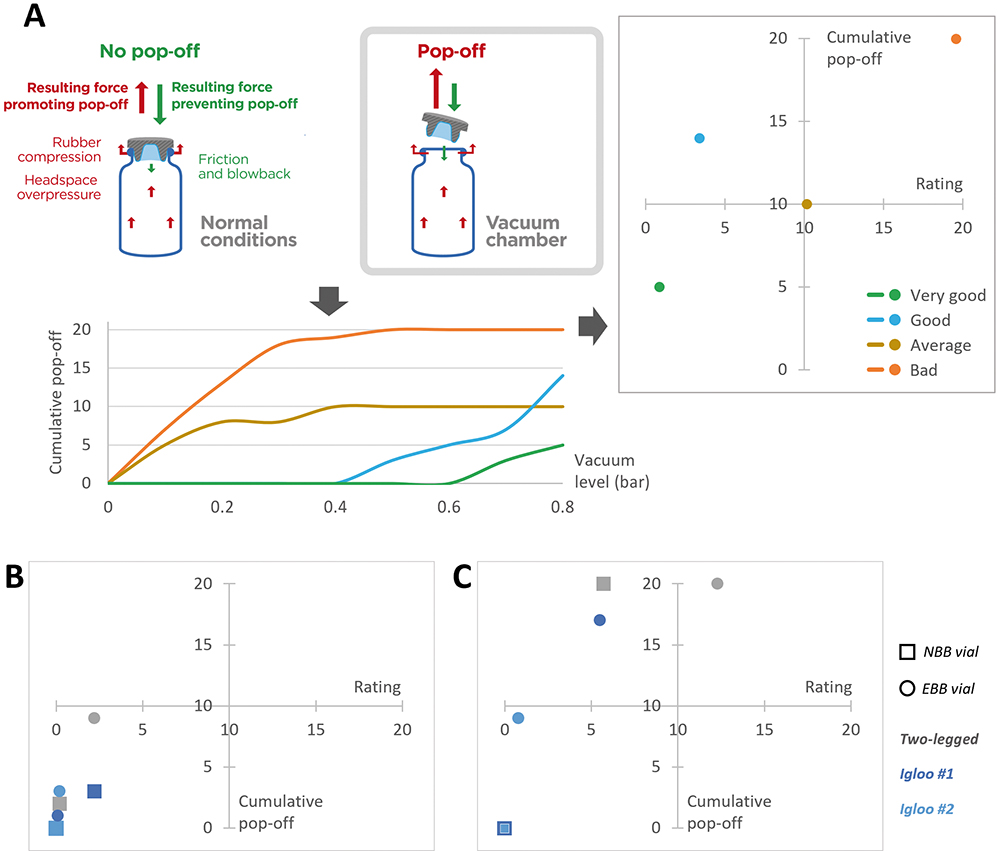
Figure 2: Evaluation of the risk of pop-off associated with various vial/stopper combinations. A: Representation of the experimental set-up and the forces at play. The curves represent the cumulative number of pop-off events with regards to the pressure gradient created in the vacuum chamber. Depending on the cumulative number of pop-off events observed and at which pressure they happen, a rating is calculated and plotted against the cumulative number of pop-off events. Representative cases were plotted to illustrate a bad, average, good and very good performance. B: Representation of performance of a two-legged, igloo #1 and igloo #2 designs for 13 mm stoppers with NBB and EBB vials. C: Representation of performance of a two-legged, igloo #1 and igloo #2 designs for 20 mm stoppers with NBB and EBB vials.
To evaluate the risk of pop-off associated with a given vial/stopper combination, Aptar Pharma’s experts have developed a specific protocol. A vial is stoppered under atmospheric conditions then placed in a vacuum chamber. The pressure is gradually decreased to increase the pressure gradient between the inside of the vial and the vacuum chamber (Figure 2A), which helps trigger pop-off artificially.
“The capacity of a stopper to maintain CCI with a given vial will directly depend on its design, as CCI relies on the establishment of an airtight seal between the glass and the rubber.”
The number of stoppers popping off, as well as the pressure difference at which they pop off, is key to properly evaluating the risk of pop-off. To assess this, Aptar Pharma calculates a rating using the integral cumulative pop-off versus the vacuum. To facilitate the visual interpretation, a matrix representation is used, the lower left quadrant representing the best performance while the top right represents suboptimal performances.
When analysing the results with 13 mm stoppers (Figure 2B), Aptar Pharma observed very strong performances overall, with all stoppers being in the lower left quadrant. The two-legged design stoppers were more likely to pop off than their counterparts, especially when combined with an EBB vial.
Figure 2C shows that the two igloo stoppers did not exhibit any pop-off when combined with an NBB vial. Some pop-off occurred when combined with the EBB vial, but the low rating indicates that pop-off only happened at higher pressure differentials. The two-legged 20 mm vial stopper popped off more frequently, especially when combined with an EBB vial. However, it is important to note that no pop-off occurred at atmospheric pressure and that the first pop-off was observed at a pressure gradient of 0.3 bar.
When choosing a vial/stopper combination, the risk of pop-off needs to be carefully considered. In the case of lyophilisation stoppers, the data demonstrate that the two-legged design is more likely to pop off than an igloo counterpart. This could be explained by the fact that, because the two legs are independent from each other, they are more susceptible to bending inward and may therefore apply less pressure on the neck off the vial, which may reduce friction.
Some manufacturers may choose two-legged designs to optimise the lyophilisation process, which can be a trade-off with the risk of pop-off when a pressure gradient builds up. However, it is important to note that this situation would only be encountered if a vial is overfilled, which cannot be the case for a lyophilised drug. Regardless of the vial design and the chosen stopper, under normal conditions, all lyophilisation stoppers are unlikely to pop off.
It is also important to emphasise the fact that the test method shown here represents an exaggeration of a worst-case scenario and that such strong pressure gradients are unlikely to happen in normal situations. None of the stoppers tested popped off with a pressure gradient below 0.3 bar.
These results demonstrate that the shape of the stoppers and the vial neck can affect the risk of pop-off. Other factors, such as the lubrication level of the stopper, may also impact the risk of pop-off and independent experiments demonstrated that reducing friction by increasing the quantity of silicone on the stoppers may lead to higher chances of pop-off (data not shown). Drug manufacturers must choose the right vial/stopper combination and manage the trade-off between reducing insertion force and reducing the risk of pop-off.
CONTAINER CLOSURE INTEGRITY
After being positioned within the vial’s neck and secured with aluminium crimping, the stopper becomes crucial in maintaining the vial’s CCI, blocking any ingress of air or germs from the external environment. The CCI is vital for the long-term preservation of the medication and avoiding contamination, thereby safeguarding patient safety. The capacity of a stopper to maintain CCI with a given vial will directly depend on its design, as CCI relies on the establishment of an airtight seal between the glass and the rubber. Storage in harsh conditions, such as deep-cold storage, and the crimping force used with the aluminium cap can also play a role, as discussed in a previous article.1
Until recently, probabilistic methods, as described in US Pharmacopeia <381>, were used. An example is methylene blue testing, where a vial is stoppered and crimped at atmospheric pressure and the system then immersed in a methylene blue solution and the pressure decreased, which would lead to a pressure gradient pulling air out of the system. The system is then brought back to ambient temperature, which leads to an ingress of methylene blue if the CCI fails, enabling visual identification.
With the implementation of USP <382> on December 1, 2025, the use of deterministic methods will become mandatory, one of which is headspace laser testing. In this test, a vial is stoppered under vacuum and stored at atmospheric pressure. A sharp increase of pressure inside the vial is indicative of CCI failure. In this second case study, featuring PremiumCoat® ETFE film-coated stoppers, this deterministic method was used.
It is important to note that blow-back vial designs were specifically designed to address pop-off related issues and not CCI issues. Therefore, when looking at the corresponding tomography pictures (Figure 3A), in every situation, the land seal is formed between the stopper’s plate and the vial’s flange, which is not affected by the vial or stopper designs. Another seal is formed between the stopper’s plug and the vial and, due to the elasticity of the rubber, it can be seen that the presence of the blow-back feature on the vial does not affect the formation of a tight seal at the top of the vial’s neck.
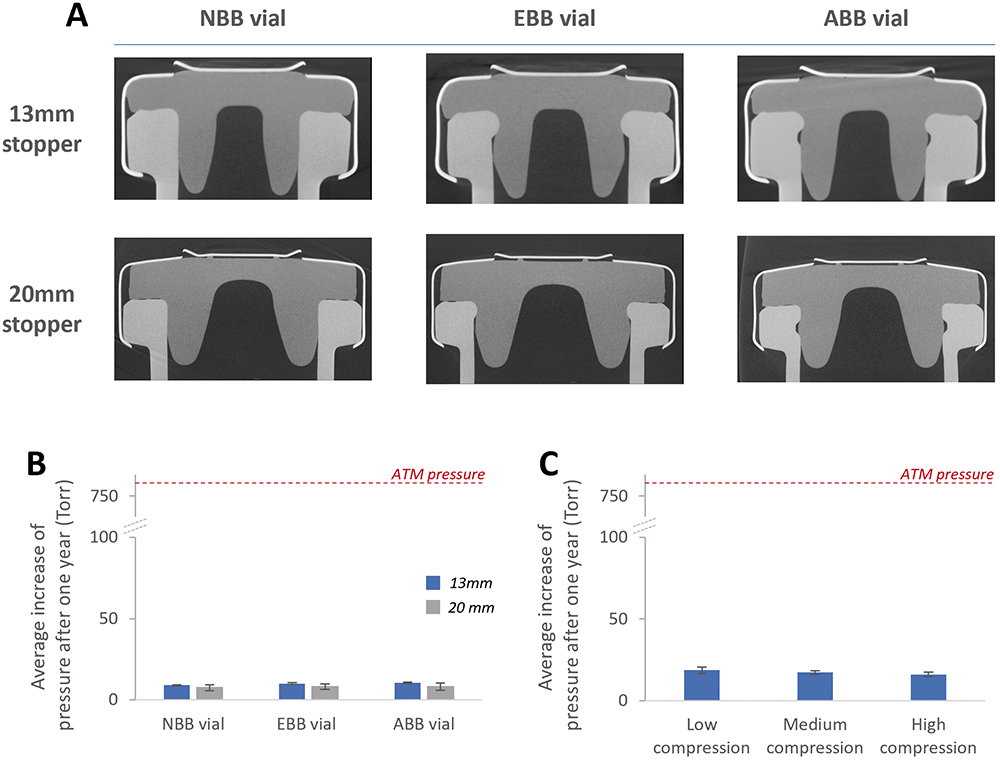
Figure 3: Evaluation of the CCI with PremiumCoat® ETFE film-coated stoppers. A: X-ray tomography of PremiumCoat® 13 or 20 mm stoppers with NBB, EBB and ABB vials. B: Evaluation of the CCI when using three different vial designs. C: Evaluation of the impact of crimping force and stopper compression on the ability of PremiumCoat® to maintain CCI.
The tomography observations were confirmed by the use of the deterministic laser headspace analysis method. Aptar Pharma’s experts investigated whether the tested stopper could maintain CCI on NBB, EBB and ABB vials after having been crimped at low, medium or high compression. Regardless of the crimping force used, Figure 3B shows that the stoppers always maintained CCI after a period of one year, despite the fact that an NBB stopper was used with EBB and ABB vials. This confirmed that the seal formed between the vial and the stoppers was able to maintain CCI for the long term.
The crimping force used to secure stoppers in place could also play a role in ensuring long-term CCI, as a higher compression could promote the formation of a tighter land seal between the stopper and the vial’s flanges. However, it is important to note that applying excessive crimping force during the stoppering process could lead to partial tearing of the rubber. Figure 3C shows that, regardless of the compression force used, the PremiumCoat® stopper tested could maintain CCI.
CONCLUSION
Vial and stopper compatibility is essential to ensure the safety of the drug and rapid time to market. The new USP <382> emphasises the responsibility of the drug manufacturer to demonstrate fitness for intended use for its primary packaging systems, instead of fully relying on the supplier. However, choosing the right partner can allow a pharma company to tap into its expertise, leverage existing data to derisk its choice and get to market faster.
Aptar Pharma has developed and tested a set of methods for evaluating the performance of its product range. Whether it is uncoated stoppers used for liquid or lyophilised applications or PremiumCoat® vial stoppers, Aptar Pharma’s designs have been demonstrated to perform appropriately across a variety of situations with a variety of vial designs. This expertise and wealth of data has allowed Aptar Pharma to create comprehensive data packages for its PremiumCoat® stoppers, which cover relevant compatibility testing (CCI, pop-off, insertion force) and a complete extractables profile for regulatory filing and simulation studies for probable leachables.
To complement Aptar Pharma’s injectables expertise, Gateway Analytical has developed a complete service offering to perform all the validation testing required for a customer’s drug filing. All tests are performed in cGMP certified labs and cover the complete range of what is required by regulatory bodies, including particle testing, extractables and leachables and CCI.
To find out more about Aptar Pharma’s injectables offering, visit: aptar.com/pharmaceutical/delivery-routes/injectables.
REFERENCE
- Cordier S, Brocco B, Verger E, Clausse A, “Cold Storage and Container Closure Integrity – Demonstrated Performance of ETFE-Coated Components.” ONdrugDelivery, Issue 147 (May 2023), pp 6–11.

